Summary
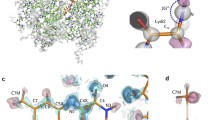
A homology model building study of cytochrome P450 2D6 has been carried out based on the crystal structure of cytochrome P450 101. The primary sequences of P450 101 and P450 2D6 were aligned by making use of an automated alignment procedure. This alignment was adjusted manually by matching α-helices (C, D, G, I, J, K and L) and β-sheets (β3/β4) of P450 101 that are proposed to be conserved in membrane-bound P450s (Ouzounis and Melvin [Eur. J. Biochem., 198 (1991) 307]) to the corresponding regions in the primary amino acid sequence of P450 2D6. Furthermore, α-helices B, B′ and F were found to be conserved in P450 2D6. No significant homology between the remaining regions of P450 101 and P450 2D6 could be found and these regions were therefore deleted. A 3D model of P450 2D6 was constructed by copying the coordinates of the residues from the crystal structure of P450 101 to the corresponding residues in P450 2D6. The regions without a significant homology with P450 101 were not incorporated into the model. After energy-minimization of the resulting 3D model of P450 2D6, possible active site residues were identified by fitting the substrates debrisoquine and dextrometorphan into the proposed active site. Both substrates could be positioned into a planar pocket near the heme region formed by residues Val370, Pro371, Leu372, Trp316, and part of the oxygen binding site of P450 2D6. Furthermore, the carboxylate group of either Asp100 or Asp301 was identified as a possible candidate for the proposed interaction with basic nitrogen atom(s) of the substrates. These findings are in accordance with a recently published predictive model for substrates of P450 2D6 [Koymans et al., Chem. Res. Toxicol., 5 (1992) 211].
Similar content being viewed by others
References
Meyer, U.A., Skoda, R.C. and Zanger, U.M., Pharmac. Ther., 46 (1990) 297.
Eichelbaum, M. and Gross, A.S., Pharmac. Ther., 46 (1990) 377.
Ayesh, R., Idle, J.R., Ritchie, J.C., Crothers, M.J. and Hetzel, M.R., Nature, 312 (1984) 169.
Kaisary, A., Smith, P., Jaczq, E., McAllister, C.B., Wilkinson, G.R., Ray, W.H. and Branch, R.A., Cancer Res., 47 (1987) 5488.
Mahgoub, A., Idle, J.R., Dring, L.G., Lancaster, R. and Smith, R.L., Lancet, ii (1977) 584.
Eichelbaum, M., Spannbrucker, N. and Dengler, H.J., In Gorrod, J. (Ed.) Biological Oxidation of Nitrogen, Elsevier/North-Holland Biomedical Press, Amsterdam, 1978, p. 113.
Wolff, T., Distlerath, L.M., Worthington, M.T., Groopman, J.D., Hammons, G.J., Kadlubar, F.F., Prough, R.A., Martin, M.V. and Guengerich, F.P., Cancer Res., 45 (1985) 2116.
Meyer, U.A., Gut, J., Kronbach, T., Skoda, C., Meier, U.T. and Catin, T., Xenobiotica, 16 (1986) 449.
Koymans, L.M.H., Vermeulen, N.P.E., VanAcker, S.A.B.E., TeKoppele, J.M., Heykants, J.J.P., Lavrijsen, K., Meuldermans, W. and Donné-Op den Kelder, G.M., Chem. Res. Toxicol., 5 (1992) 211.
Ferenczy, G.G. and Morris, G.M., J. Mol. Graph., 7 (1989) 206.
Laughton, C.A., Neidle, S., Zvelebil, M.J.J.M. and Sternberg, M.J.E., Biochem. Biophys. Res. Commun., 171 (1990) 1160.
Zvelebil, M.J.J.M., Wolf, C.R. and Sternberg, M.J.E., Protein Eng., 4 (1991) 271.
Graham-Lorence, S., Khali, M.W., Lorence, M.C., Mendelson, C.R. and Simpson, E.R., J. Biol. Chem., 266 (1991) 11939.
Chotia, C. and Lesk, A.M., EMBO J., 5 (1986) 823.
Koymans, L.M.H., Grootenhuis, P.D.J. and Haasnoot, C.A.G., Recl. Trav. Chim. Pays Bas (1993) in press.
Thornton, J.M., Sibanda, B.L., Edwards, M.S. and Barlow, D.J., BioEssays, 8 (1988) 63.
Tramontano, A., Chotia, C. and Lesk, A.M., Proteins, 6 (1989) 382.
Nelson, D.R. and Strobel, H.W., Biochemistry, 28 (1989) 656.
Edwards, R.J., Murray, B.P., Boobis, A.R. and Davies, D.S., Biochemistry, 28 (1989) 3762.
Ouzounis, C.A. and Melvin, T., Eur. J. Biochem., 198 (1991) 307.
Black, S.D. and Coon, M.J., In Ortiz de Montellano, P.R. (Ed.) Cytochrome P-450 Structure, Mechanism and Biochemistry, Plenum Press, New York, 1986, p. 161.
Nelson, D.R. and Strobel, H.W., Mol. Biol. Evol., 4 (1987) 572.
Nelson, D.R. and Strobel, H.W., J. Biol. Chem., 263 (1988) 6038.
Gotoh, O. and Fujii-Kuriyama, Y., In Ruckpaul, K. and Rein, H. (Eds.) Frontiers in Biotransformation, Vol. 1, Akademie Verlag, Berlin, 1989, p. 195.
Bernstein, F.C., Koetzle, T.F., Williams, G.J.B., Meyer, E.F., Brice, M.D., Rodgers, J.R., Kennard, O., Shimanouchi, T. and Tasumi, M., J. Mol. Biol., 112 (1977) 535.
Meyers, E. and Miller, W., CABIOS, 4 (1988) 11.
Pearson, W.R. and Lipman, D.J., Proc. Natl. Acad. Sci. USA, 85 (1988) 2444.
Poulos, T.L., Finzel, B.C. and Howard, A.J., J. Mol. Biol., 195 (1987) 687.
Ishida, N., Aoyama, Y., Hatanaka, R., Oyama, Y., Imajo, S., Ishiguro, M., Oshima, T., Nakazato, H., Noguchi, T., Maitra, U.S., Mohan, V.P., Sprinson, D.B. and Yoshida, Y., Biochem. Biophys. Res. Commun., 155 (1988) 317.
Poulos, T.L., Finzel, B.C., Gunsalus, I.C., Wagner, G.C. and Kraut, J., J. Biol. Chem., 260 (1985) 16122.
Lesk, A.M. and Chotia, C., J. Mol. Biol., 136 (1980) 225.
Lewis, D.F.V. and Moereels, H., J. Comput.-Aided Mol. Design, 6 (1992) 235.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koymans, L.M.H., Vermeulen, N.P.E., Baarslag, A. et al. A preliminary 3D model for cytochrome P450 2D6 constructed by homology model building. J Computer-Aided Mol Des 7, 281–289 (1993). https://doi.org/10.1007/BF00125503
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00125503