Summary
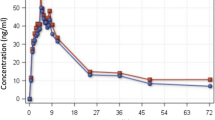
Plasma quinine concentrations following oral quinine sulphate 10 mg salt/kg have been measured by HPLC in 15 adult Thai patients with uncomplicated falciparum malaria. In 10 of the same patients the study was repeated in convalescence. In acute malaria plasma concentrations were approximately 50% higher than in convalescence; the mean acute peak plasma quinine concentration was 8.4 mg·l−1 compared to 5.7 mg·l−1 in convalescence.
There was considerable variation in the rate of drug absorption, particularly in acute malaria. The mean time to peak plasma concentration was 5.9 h in acute malaria and 3.2 h in convalescence. The apparent clearance of oral quinine (CL/f) during the illness was 1.51 ml·kg−1·min−1, which was significantly lower than in convalescence — 2.67 ml·kg−·min−1. Estimated free quinine clearance was also lower in the acute phase: 30.6 compared to 49.0 ml·kg−1·min−1 in convalescence. Mean (SD) plasma protein binding of quinine was 94.7% in acute malaria and 92.8% in convalescence. Binding was significantly correlated with the plasma concentration of α1 acid glycoprotein (r=0.5), which was significantly higher in the acute phase; 1.48 g·l−1 compared to 1.05 g·l−1 during convalescence.
Oral quinine sulphate was well absorbed in uncomplicated falciparum malaria. High blood concentrations following the administration of oral quinine in acute malaria are probably related to increased plasma protein binding, lower apparent volume of distribution, and a reduction in its systemic clearance.
Similar content being viewed by others
References
Brooks MH, Malloy JP, Bartelloni P, Sheehy TW, Barry KG (1969) Quinine pyrimethamine and sulphorthodimethoxine. Clinical response, plasma levels and urinary excretion during the initial attack of naturally acquired falciparum malaria. Clin Pharmacol Ther 10: 85–91
Edstein M, Stace J, Shann F (1983) Quantification of quinine in human serum by high performance liquid chromatography. J Chromatogr 278: 445–451
Garnham JC, Raymond D, Shotton E, Turner P (1976) The bioavailability of quinine. J Trop Med Hyg 70: 264–269
Hall AP, Hanchalay S, Doberstyn EB, Bumnetphund S (1975) Quinine dosage and serum levels in falciparum malaria. SEATO Annual Progress Report of the Research Laboratories, Bangkok, pp240–250
Jamaludin A, Mohamad M, Navaratnam V, Selliah K, Tan SC, Wernsdorfer WH, Yuen KH (1988) Relative bioavailability of the hydrochloride, sulphate and ethylcarbonate salts of quinine. Br J Clin Pharmacol 25: 261–263
Karbwang J, Molunto P, Na Bangchang K, Bunnag D (1989) Determination of quinine and quinidine in biological fluids by high performance liquid chromatography. Southeast Asian J Trop Med Public Health 20: 55–60
Shann F, Stace J, Edstein M (1985) Pharmacokinetics of quinine in children. J Pediatr 106: 506–510
Silamut K, White NJ, Looareesuwan S, Warrell DA (1985) Binding of quinine to plasma proteins in falciparum malaria. Am J Trop Med Hyg 34: 681–686
Taggart JV, Earle DP, Berliner RW et al. (1948) Studies on the chemotherapy of the human malarias II. The physiological disposition and antimalarial activity of the cinchona alkaloids. J Clin Invest 27: 80–86
Trenholme GM, Williams RL, Rieckman KH, Frischer H, Carson PE (1976) Quinine disposition during malaria and during induced fever. Clin Pharmacol Ther 19: 459–467
White NJ (1987) The pharmacokinetics of quinine and quinidine in malaria. Acta Leidensia 55: 65–76
White NJ (1988) Drug treatment and prevention of malaria. Eur J Clin Pharmacol 34: 1–14
White NJ, Looareesuwan S, Warrell DA, Warrell MJ, Bunnag D, Harinasuta T (1982) Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med 73: 564–672
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Supanaranond, W., Davis, T.M.E., Pukrittayakamee, S. et al. Disposition of oral quinine in acute falciparum malaria. Eur J Clin Pharmacol 40, 49–52 (1991). https://doi.org/10.1007/BF00315138
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315138