Abstract
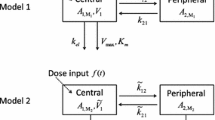
A dispersion model developed in Chromatographic theory is applied to the analysis of the elution profile in the liver perfusion system of experimental animals. The equation for the dispersion model with the linear nonequilibrium partition between the perfusate and an organ tissue is derived in the Laplace-transformed form, and the fast inverse Laplace transform (FILT) is introduced to the pharmacokinetic field for the manipulation of the transformed equation. By the analysis of the nonlinear least squares method associated with FILT, this model (two-compartment dispersion model) is compared to the model with equilibrium partition between the perfusate and the liver tissue (one-compartment dispersion model) for the outflow curves of ampicillin and oxacillin from the rat liver. The model estimation by Akaike's information criterion (AIC) suggests that the two-compartment dispersion model is more proper than the one-compartment dispersion model to mathematically describe the local disposition of these drugs in the perfusion system. The blood space in the liver, VB, and the dispersion number DN are estimated at 1.30 ml (±0.23 SD) and 0.051 (±0.023 SD), respectively, both of which are independent of the drugs. The efficiency number, RN, of ampicillin is 0.044 (±0.049 SD) which is significantly smaller than 0.704 (±0.101 SD) of oxacillin. The parameters in the two-compartment dispersion model are correlated to the recovery ratio, FH, mean transit time, ¯tH, and the relative variance, σ2/¯tH 2, of the elution profile of drugs from the rat liver.
Similar content being viewed by others
Abbreviations
- A :
-
Cross-sectional area of the blood space
- C(t, z) :
-
Concentration of drug (one-compartment dispersion model)
- ∼C(s, z) :
-
Laplace transform of C(t, z)
- C 1(t, z):
-
Concentration of drug in blood space (two-compartment dispersion model)
- C 2(t, z):
-
Concentration of drug in the liver tissue (two-compartment dispersion model)
- ∼C 1 (s, z):
-
Laplace transform ofC 1(t, z)
- D :
-
Axial or longitudinal dispersion coefficient
- D c(=D· A 2):
-
Corrected dispersion coefficient
- D N :
-
Dispersion number
- f I(t):
-
Input function with respect tot
- fI(z):
-
Input function with respect toz
- FI(s):
-
Laplace transform of fI(t)
- fs(t):
-
System weight function with respect tot
- fs(z):
-
System weight function with respect to z
- FH :
-
Recovery ratio
- k′ :
-
Partition ratio (distribution ratio)
- k12, k21 :
-
Forward and backward partition rate constant in the central elimination two-compartment dispersion model
- k p12 ,k p21 :
-
Forward and backward partition rate constant in the peripheral elimination two-compartment dispersion model
- ke :
-
Elimination (or irreversible transfer) rate constant
- k pe :
-
Elimination rate constant in peripheral elimination model
- KH :
-
Distribution constant
- L :
-
Length of blood space in liver
- M :
-
Amount of drug injected
- m :
-
Coefficient related to the injected amount
- ph :
-
Mass transfer coefficient from perfusate to hepatic tissue
- Q :
-
Flow rate of perfusate
- RN :
-
Efficiency number
- s :
-
Laplace variable
- t :
-
Time
- ¯ tH :
-
Mean transit time
- υ :
-
Linear flow velocity of the perfusate
- V B(= L·A):
-
Blood volume (sum of the sinusoid volume and the space of Disse)
- vh :
-
Apparent volume of distribution
- V ′H :
-
Anatomical volume of liver tissue
- z :
-
Axial coordinate in the liver
- δ(t):
-
Delta function
- ɛ :
-
Volume ratio of the anatomical liver tissue to the blood space
- δ2 :
-
Variance of transit time
- δ2/¯t 2H :
-
Relative dispersion to transit time
- ∂ :
-
Partial derivatives
References
K. S. Pang and M. Rowland. Hepatic clearance of drugs: 1. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance.J. Pharmacokin. Biopharm. 5:625–653 (1977).
G. R. Wilkinson and D. G. Shand. Commentary. A physiological approach to hepatic drug clearance.Clin. Pharmacol. Ther. 18:377–390 (1975).
M. Rowland, L. Z. Benet, and G. G. Graham. Clearance concepts in pharmacokinetics.J. Pharmacokin. Biopharm. 1:123–136 (1977).
L. Bass, S. Keiding, K. Winkler, and N. Tygstrup. Enzymatic elimination of substrates flowing through the intact liver.J. Theor. Biol. 61:393–409 (1976).
R. W. Brauer, G. F. Leong, P. F. McElory, Jr., and R. J. Holloway. Circulatory pathways in the rat liver as revealed by32P chromic phosphate colloid uptake in the isolated perfused liver preparation.Am. J. Physiol. 184:593–598 (1956).
M. Weiss. Moment of physiological transit time distribution and the time course of drug disposition in the body.J. Math. Biol. 15:305–318 (1982).
L. Bass, P. Robinson, and A. J. Bracken. Hepatic elimination of flowing substrates: the distributed model.J. Theor. Biol. 72:161–184 (1978).
E. L. Forker and B. Luxton. Hepatic transport kinetic and plasma disappearance curves: Distributed modeling versus conventional approach.Am. J. Physiol. 235:E648-E660 (1978).
C. A. Goresky, W. H. Ziegler, and G. G. Bach. Capillary exchange modeling. Barrier-limited and flow limited distribution.Circ. Res. 27:739–764 (1970).
M. S. Roberts and M. Rowland. Hepatic elimination: dispersion model.J. Pharm. Sci. 74:585–587 (1985).
M. S. Roberts and M. Rowland. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations.J. Pharmacokin. Biopharm. 14:227–260 (1986).
L. Lapidus and N. R. Amundson. Mathematics of adsorption in beds. VI. The effect of longitudinal diffusion in ion exchange and Chromatographic columns.J. Phys. Chem. 56:984–988 (1952).
K. Yamaoka, and T. Nakagawa. Moment analysis for reaction chromatography.J. Chromatog. 117:1–10 (1976).
T. Hosono. Numerical inversion of Laplace transform and some applications to wave optics. Radio Sci.16:1015–1019 (1981).
K. Yamaoka, Y. Tanigawara, T. Nakagawa, and T. Uno. A pharmacokinetic analysis program (MULTI) for microcomputer.J. Pharmacobio-Dyn. 4:879–885 (1981).
A. B. Littlewood.Gas Chromatography, Academic Press, New York, 1970, pp. 164–232.
O. Levenspiel.Chemical Reaction Engineering, Wiley, New York, 1972, pp. 235–315.
C. A. Goresky and G. G. Bach. Membrane transport and the hepatic circulation.Ann. N.Y. Acad. Sci. 170 (1):18–47 (1970).
K. Yamaoka, T. Nakagawa, and T. Uno. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations.J. Pharmacokin. Biopharm. 6:165–175 (1978).
M. Barza and L. Weinstein. Pharmacokinetics of the penicillins in man.Clin. Pharmacokin. 1:297–308 (1976).
Y. Murai, T. Nakagawa, K. Yamaoka, and T. Uno. High performance liquid Chromatographic analysis and pharmacokinetic investigation of oxacillin and its metabolites in man.Chem. Pharm. Bull. 29:3290–3297 (1981).
C. A. Goresky. A linear method for determining liver sinusoidal and extravascular volumes.Am. J. Physiol. 204:626–640 (1963).
W. Perl and F. P. Chinard. A convection-diffusion model of indicator transport through an organ.Circ. Res. 22:273–298 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yano, Y., Yamaoka, K., Aoyama, Y. et al. Two-compartment dispersion model for analysis of organ perfusion system of drugs by fast inverse laplace transform (FILT). Journal of Pharmacokinetics and Biopharmaceutics 17, 179–202 (1989). https://doi.org/10.1007/BF01059027
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059027