Abstract
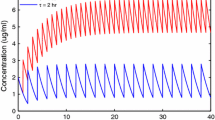
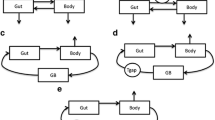
The enterohepatic circulation of cefixime in rat was evaluated by a nonlinear least square analysis program, MULTI(FILT), into which the fast inverse Laplace transform (FILT) was incorporated. The plasma time course in the bile duct-cannulated rat exhibited a biexponential curve after the rapid iv administration of cefixime. Several pharmacokinetic models for the enterohepatic circulation were constructed based on the recirculatory concept and the Laplace-transformed equations corresponding to these models were derived by means of the method of transfer function. The transformed equations were simultaneously fitted to the time courses of plasma concentration in rats with laparotomy and with bile duct cannula. The optimum model was selected based on the Akaike's information criterion (AIC). The local moment characteristics for a single pass through enterohepatic circulation were further calculated from the time courses of both the plasma concentration and the amount excreted into the bile. The recovery ratio (Fc and the mean circulatory time (¯tc through a single pass of enterohepatic circulation were estimated 27.9% and 1.07 hr, respectively. The recovery ratio (Fa and the mean absorption time (¯ta for the absorption process from the intestinal tract into the systemic circulation were 68.3% and 0.0234 hr, respectively. The recovery ratio (Fb and the mean transit time (¯tb)for the disposition process through the systemic circulation into the bile were 40.8% and 1.05hr, respectively.
Similar content being viewed by others
Abbreviations
- A i :
-
coefficient
- a i :
-
exponent
- \(\tilde C_p^{iv} \)(s):
-
Laplace transform of the time course of plasma following intravenous dose
- \(\tilde C_p^{po} \)(s):
-
Laplace transform of the time course of plasma following oral administration dose
- \(\tilde C_p^1 \)(s):
-
Laplace transform of the time course of plasma concentration without EHC
- CLb :
-
clearance into the bile
- CL1 :
-
total clearance through the single EHC (=CLb/Fb)
- Div :
-
intravenous dose
- Dpo :
-
oral administration dose
- Fa :
-
recovery (availability) from intestinal tract to systemic circulation
- Fb :
-
recovery from systemic circulation to intestinal tract
- F′a :
-
recovery from oral dose (absolute availability)
- Fc :
-
recovery through a single pass of EHC
- Fg :
-
recovery through the stomach
- \(\tilde f_a \)(s):
-
transfer function corresponding to the process outside the body through the intestinal tract
- \(\tilde f_a^\prime \)(s):
-
transfer function for oral dose
- \(\tilde f_b \)(s):
-
transfer function through the systemic circulation into the bile
- \(\tilde f_c \)(s):
-
transfer function for a single pass of EHC
- \(\tilde f_g \)(s):
-
transfer function through the stomach
- fi(t):
-
weight function for the processi
- \(\tilde f_i \)(s):
-
Laplace transform off t (t)
- \(\tilde f_r \)(s):
-
transfer function corresponding to the recirculatory process
- ka :
-
absorption rate constant
- s :
-
Laplace variable
- \(\bar t_a \) :
-
mean transit time for the absorption process from the intestinal tract
- \(\bar t_a^\prime \) a :
-
mean transit time for oral dose (=MAT)
- \(\bar t_b \) :
-
mean transit time for the disposition process in the body
- \(\bar t_c \) :
-
mean transit time for a single pass of EHC
- t 0 :
-
gap time
References
P. V. Pedersen and R. Miller. Pharmacokinetics and bioavailability of cimetidene in humans.J. Pharm. Sci. 69:394–398 (1980).
P. V. Pedersen and R. Miller. Pharmacokinetics of doxycline reabsorption.J. Pharm. Sci. 69:204–207 (1980).
F. L. S. Tse, F. Ballard, J. M. Jaffe, and H. J. Schwarz, Enterohepatic circulation of radioactivity following an oral dose of [14C]temazeparn in the rat.J. Pharm. Pharmacol. 35:225–228 (1983).
B. E. Dahlstrom and L. K. Paalzow. Pharmacokinetic interpretation of the enterohepatic recirculation and first-pass elimination of morphine in the rat.J. Pharmacokin. Biopharm. 6:505–519 (1978).
K. Singh, J. M. Orr, and F. S. Abbott. Pharmacokinetics and enterohepatic circulation of 2-n-propyl-4-pentenoic acid in the rat.Drug Metab. Dispos. 16:848–852 (1988).
N. Watari, M. Hanawa, M. Iwai, and N. Kaneniwa.J. Pharmacobiodyn. 7:811–819 (1984).
T. Tsuchida, M. Terakawa, K. Ishibashi, H. Noguchi, and R. Kato.Arzneim. Forsch. 30:1650–1653 (1980).
T. A. Shepard, R. H. Reuning, and L. J. Aarons. Interpretation of area under the curve measurements for drugs subject to enterohepatic cycling.J. Pharm. Sci. 74:227–228 (1985).
K. S. Pang and J. R. Gillette. A theoretical examination of the effects of gut wall metabolism, hepatic elimination, and enterohepatic recycling on estimates of bioavailability and of hepatic blood flow.J. Pharmacokin. Biopharm. 6:355–367 (1978).
F. L. S. Tse, F. Ballard, and J. Skinn. Estimating the fraction reabsorbed in drugs undergoing enterohepatic circulation.J. Pharmacokin. Biopharm. 10:455–460 (1982).
T. A. Shepard, G. F. Lockwood, L. J. Aarons, and I. D. Abrahams. Mean residence time for drugs subject to enterohepatic cycling.J. Pharmacokin. Biopharm. 17:327–345 (1989).
A. F. Hofmann, C. Cravetto, G. Molino, G. Belforte, and B. Bona. Simulation of the metabolism and enterohepatic circulation of endogenous deoxycholic acid in humans using a physiologic pharmacokinetic model for bile acid metabolism.Gastroenterology 93:693–709 (1987).
T. A. Shepard, D. J. Gannaway, and G. F. Lockwood. Accumulation and time to steady state for drugs subject to enterohepatic cycling: A simulation study.J. Pharm. Sci. 74:1331–1333 (1985).
W. A. Colburn. Pharmacokinetic analysis of concentration-time data obtained following administration of drugs that are recycled in the bile.J. Pharm. Sci. 73:313–317 (1984).
Y. Yano, K. Yamaoka, and H. Tanaka. A nonlinear least squares program, MULTI(FILT), based on fast inverse Laplace transform for microcomputers.Chem. Pharm. Bull 37:1035–1038 (1989).
T. Hosono. Numerical inversion of Laplace transform and some applications to wave optics.Radio Sci. 16:1015–1019 (1981).
H. Yamanaka, Y. Chiba, K. Kawabata, H. Takasugi, T. Masugi, and T. Takaya. Study ofβ-lactam antibiotics synthesis and biological activity of a new orally active cephalosporin, cefixime (FK027).J. Antibiot. 38:1738–1751 (1985).
Y. Tokuma, M. Sekiguti, T. Fujiwara, and H. Noguchi. Absorption, distribution, excretion and metabolism of cefixime in rats.Xenobio. Metab. Dispos. 2:673–648 (1987).
H. Sakamoto, S. Hirose, Y. Mine, S. Gosima, M. Nisida, and S. Kuwahara. Pharmacokinetics of cefixime, a new oral cephalosporin, in experimental animals.Chemotherapy (Japan)33:(S6): 157–167 (1985).
M. Weiss. Moments of physiological transit time distributions and the time course of drug disposition in the body.J. Math. Biol. 15:305–318 (1982).
K. Yamaoka, T. Nakagawa, and T. Uno. Statistical moment in pharmacokinetics.J. Pharmacokin. Biopharm. 6:547–558 (1978).
T. Kakutani, K. Yamaoka, M. Hashida, and H. Sezaki. A new method for assessment of drug disposition in muscle: application of statistical moment theory to local perfusion systems.J. Pharmacokin. Biopharm. 13:609–631 (1985).
Y. Yano, K. Yamaoka, Y. Aoyama, and H. Tanaka. Two-compartment dispersion model for analysis of organ perfusion system of drugs by fast inverse Laplace transform (FILT).J. Pharmacokin. Biopharm. 17:179–202 (1989).
K. Yamaoka, T. Nakagawa, and T. Uno. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetics equations.J. Pharmacokin. Biopharm. 6:165–175 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaoka, K., Kanba, M., Toyoda, Y. et al. Analysis of enterohepatic circulation of cefixime in rat by fast inverse Laplace transform (FILT). Journal of Pharmacokinetics and Biopharmaceutics 18, 545–559 (1990). https://doi.org/10.1007/BF01073938
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01073938