Abstract
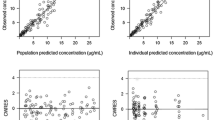
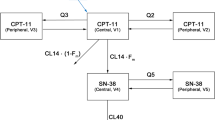
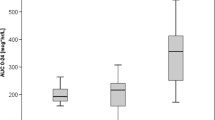
Pharmacokinetic parameters of antineoplastic drugs are usually generated from concentration/time profiles obtained after multiple venipunctures. With limited-sampling models (LSM) this number can be reduced to between one and three timed plasma samples. LSMs may facilitate population pharmacokinetic/pharmacodynamic studies, which eventually may lead to a dosing strategy based on the characteristics of the individual patient. In this article, the development, validation and application of several LSMs reported in the literature are reviewed.
Similar content being viewed by others
Abbreviations
- LSM:
-
limited-sampling model
- AUC:
-
area under the concentration/time curve
References
Ackland SP, Choi KE, Ratain MJ, Egorin MJ, Williams SF, Sinkule JA, Bitran JD (1988) Human plasma pharmacokinetics of thiotepa following administration of high-dose thiotepa and cyclophosphamide. J Clin Oncol 6:1192–1196
Adamson PC, Balis FM, Miser J, Arndt C, Wells RJ, Gillespie A, Aronson L, Penta JS, Clendeninn NJ, Poplack DG (1992) Pediatric phase I trial, pharmacokinetic study, and limited sampling strategy for piritrexim administered on a low-dose, intermittent schedule. Cancer Res 52:521–524
Bennet CL, Sinkule JA, Schilsky RL, Senekjian E, Choi KE (1987) Phase I clinical and pharmacological study of 72-hour continuous infusion of etoposide in patients with advanced cancer. Cancer Res 47:1952–1956
Bressolle F, Ray P, Jacquet JM, Brès J, Galtier M, Donadio D, Jourdan J, Rossi JF (1991) Bayesian estimation of doxorubicin pharmacokinetic parameters. Cancer Chemother Pharmacol 29:53–60
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–56
CD-ROM MEDLINE (1993) Compact Cambridge, Wisconsin Ave, Bethesda, USA
Collins JM, Zaharko DS, Dedrick RL, Chabner BA (1986) Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat Rep 70:73–80
Conley BA, Forrest A, Egorin MJ, Zuhowski EG, Sinibaldi V, Van Echo DA (1989) Phase I trial empolying adaptive control dosing of hexamethylene bisacetammide (HMBA, NCS 95580). Cancer Res 49:3436–3440
Cooper MR, Lieberman R, LaRocca RV, Gernt PR, Weinberger RN, Headle RN, Kohler DR, Goldspiel BR, Peck CC, Meyers CE (1992) Adaptive control with feedback strategies for suramin dosing. Clin Pharmacol Ther 52:11–23
Desai ZR, Van den Berg HW, Bridges JM, Shanks RG (1982) Can severe vincristine neurotoxicity be prevented? Cancer Chemother Pharmacol 8:211–214
Drusano GL, Forrest A, Snyder MJ, Reed MD Blumer JL (1988) An evaluation of optimal sampling strategy and adaptive study design. Clin Pharmacol Ther 44:232–8
Egorin MJ, Van Echo DA, Tipping SJ, Olman EA, Whitacre MY, Thompson BW, Aisner J (1984) Pharmacokinetics and dosage reduction ofcis-diamine(1,1-cyclobutannedicarboxylato) platinum in patients with impaired renal function. Cancer Res 44:5432–5438
Egorin MJ, Van Echo DA, Whitacre MY, Forrest A, Sigman LM, Enlish KL, Aisner J (1986) Human pharmacokinetics, excretion, and metabolism of the anthracycline antibiotic menogoril (7-OMEN, NSC 269148) and their correlation with clinical toxicities. Cancer Res 46:1513–1520
Egorin MJ, Conley BA, Forrest A, Zuhowski EG, Sinibaldi V, Van Echo DA (1987) Phase I study and pharmacokinetics of menogaril (NSC 269148) in patients. Cancer Res 47:6104–6110
Egorin MJ, Forrest A, Belani CP, Ratain M, Abrams JS Van Echo DA (1989) A limited sampling strategy for cyclophosphamide pharmacokinetics. Cancer Res 49:3129–3133
Egorin MJ (1992) Therapeutic drug monitoring and dose optimisation in oncology. In Workman P (ed) New approaches in cancer pharmacology: drug design and development. Springer, Berlin Heidelberg New York, pp 75–91
Eichholtz-Wirth H (1980) Dependence of the cytostatic effect of Adriamycin on drug concentration and exposure time in vitro. Br J Cancer 41:886–891
Eksorg S (1990) Anthracycline pharmacokinetics. Limited sampling model for plasma level monitoring with special reference to epirubicin (Farmorubicin). Acta Oncol 29:339–342
EORTC Pharmacokinetics and Metabolism Group (1987) Pharmacokinetically guided dose esclation in phase I clinical trials. Commentary and proposed guidelines. Eur J Clin Oncol 23:1083:1087
Evans WE, Relling MV (1989) Clinical pharmacokinetics-pharmacodynamics of anticancer drugs. Clin Pharmacokinet 16:327–336
Evans WE, Rodman J, Relling MV, Crom WR, Rivera GK, Crist WM, Pui CH (1991) Individualized dosages of chemotherapy as a strategy to improve response for acute lymphocytic leukemia. Semin Hematol 28:15–21
Gibaldi M, Perrier D (1982). In: Pharmacokinetics, 2nd edn. Dekker, New York Basel
Goldberg JA, Kerr DJ, Willmott N, McKillop JH, McArdle CS (1988) Pharmacokinetics and pharmacodynamics of locoregional 5-fluorouracil (5FU) in advanced colorectal liver metastases. Br J Cancer 57:186–189
Grochow LB, Colvin M (1979) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 4:380–394
Hamilton SF, Evans WE (1981) Accuracy of using pre- and post-dose gentamycin serum concentrations to estimate pharmacokinetics parameters and adjust doses in children and adolescents. Ther Drug Monit 3:57–61
Jakobsen P, Steiness E, Bastholt L, Dalmark M, Lorenzen A, Petersen D, Gjedde SB, Sandberg E, Rose C, Nielsen OS, Mouridsen HT, Jakobsen A (1991) Multiple-dose pharmacokinetics of epirubicin at four different dose levels: studies in patients with metastatic breast cancer. Cancer Chemother Pharmacol 28:63–68
Jelliffe RW, Schumitzky A, Van Guilder M, Liu M, Hu L, Maire P, Gomis P, Barbaut X, Tahani B (1993) Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting and adaptive control. Ther Drug Monit 15:380–393
Jodrell DI, Egorin MJ, Canetta RM, Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S, Wiltshaw E (1992) Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 10:520–528
Joel SP, Heap L, Robbins S, Clarke PI, Slevin ML (1990) A limited sampling strategy for the calculation of etoposide pharmacokinetics. Proc Am Assoc Clin Oncol 9:67
Koup JR, Sack CM, Smith AL, Gibaldi M (1979) Hypothesis for the individualisation of drug dosage. Clin Pharmacokinet 4:460–9
LaRocca RV, Meer J, Gilliatt RW, Stein CA, Cassidy J, Myers CE (1990) Suramin-induced polyneuropathy. Neurology 40:954–960
Launay MC, Milano G, Iliadis A, Frenay M, Namer N (1989) Limited sampling procedure for estimating adriamycin pharmacokinetics in cancer patients. Br J Cancer 60:89–92
Liliemark J, Peterson C (1991) Pharmacokinetic optimisation of anti-cancer therapy. Clin Pharmacokinet 21:213–231
Maes RAA, Oellerich M (1988) In: Tobramycin profile. Report IX of the Senate Commission for Clinical-Toxological Analysis, DFG. VCH Verlagsgesellschaft, Weinheim, pp 1–30
Mick R, Ratain MJ (1991) Modeling interpatient pharmacodynamic variability of etoposide. J Natl Cancer Inst 83:1560–1564
Miller AA, Tolley EA, Niell HB, Stewart CF, Griffin JP (1992) Pharmacokinetics of three daily infusions of etoposide in patients with extensive-stage small-cell lung cancer. Cancer Chemother Pharmacol 31:161–166
Moore MJ, Erlichman C (1987) Therapeutic drug monitoring in oncology: problems and potential in antineoplastic therapy. Clin Pharmacokinet 13:205–227
Moore MJ, Bunting P, Yuan S, Thiessen JJ (1993) Development and validation of a limited sampling strategy for 5-fluorouracil given by bolus intravenous administration. Ther Drug Monit 15:394–399
Port RE, Edler L, Herrman R, Feldmann U (1991) Pharmacokinetics of 5-fluorouracil after short systemic infusion: plasma level at the end of the distribution phase as an indicator of the total area under the plasma concentration-time curve. Ther Drug Monit 13:96–102
Ratain MJ, Staubus AE, Schilsky RL, Malspeis L (1988) Limited sampling models for amonafide (NSC 308847) pharmacokinetics. Cancer Res 48:4127–4130
Ratain MJ, Vogelzang NJ (1987) Limited sampling model for vinblastine pharmacokinetics. Cancer Treat Rep 71:935–939
Ratain MJ, Schilsky RL, Choi KE, Guarnieri C, Grimmer D, Vogelzang NJ, Senekjian E, Liebner (1989) Adaptive control of etoposide dosing: impact of interpatient pharmacodynamic variability. Clin Pharmacol Ther 45:226–233
Ratain MJ, Schilsky RL, Conley BA, Egorin MJ (1990) Pharmacodynamics in cancer therapy. J Clin Oncol 8:1739–1753
Ratain MJ, Mick R, Schilsky RL, Vogelzang NJ, Berezin F (1991a) Pharmacologically based dosing of etoposide: a means of safely increasing dose intensity. J Clin Oncol 9:1480–1486
Ratain MJ, Robert J, Vijgh WJF van der (1991b) Limited sampling models for doxorubicin pharmacokinetics. J Clin Oncol 9:871–6
Robert J (1993) Use of pharmacokinetic-pharmacodynamic relationships in the development of new anthracyclines. Cancer Chemother Pharmacol 32:99–102
Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, Schneider M, Demard F (1989) 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 59:287–290
Scher HI, Jodrell DI, Iversen JM, Curley T, Tong W, Egorin MJ, Forrest A (1992) Use of adaptive control with feedback to individualize suramin dosing. Cancer Res 52:64–70
Schumacher GE (1985) Choosing optimal sampling times for therapeutic drug monitoring. Clin Pharmacol 4:84–92
Scheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharmacol 9:503–512
Sørensen BT, Strömgren A, Jakobsen P, Jakobsen A (1993) A limited sampling method for estimation of the carboplatin area under the curve. Cancer Chemother Pharmacol 31:324–327
Spector R, Park GD, Johnson GF, Vesell ES (1988) Therapeutic drug monitoring. Clin Pharmacol Ther 43:345–353
Stewart CF, Arbuck SG, Fleming RA, Evans WE (1991) Relation of systemic exposure to unbound etoposide and hematologic toxicity. Clin Pharmacol Ther 50:385–393
Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA (1977) Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med 297:630–634
Strömgren A, Sørensen BT, Jakobsen A (1993) A limited sampling method for estimation of the etoposide area under the curve. Cancer Chemother Pharmacol 32:226–230
Sulkes A, Collins JM (1987) Reapraisal of some dosage adjustment guidelines Cancer Treat Rep 71:229–33
Thomson AH, Whiting B (1992) Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet 22:447–467
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Warmerdam, L.J.C., ten Bokkel Huinink, W.W., Maes, R.A.A. et al. Limited-sampling models for anticancer agents. J Cancer Res Clin Oncol 120, 427–433 (1994). https://doi.org/10.1007/BF01240143
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01240143