Abstract
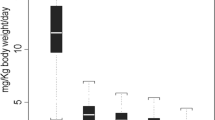
To assess the incidence of cyclosporine A-induced hepatotoxicity, we retrospectively analyzed liver biochemical test results in 59 patients with endogenous uveitis who received cyclosporine A. All patients had normal liver tests before treatment and had at least six determinations during a 6- to 36-month course of therapy with cyclosporine A at a dose of 2–10 mg/kg/day. Thirty-four (58%) patients developed at least one abnormality of liver tests, and 19 (32%) had a prolonged pattern of abnormalities. The usual abnormalities consisted of a mild, transient increase in alkaline phosphatase levels occasionally accompanied by slight elevations in serum bilirubin and aminotransferase activities. Peak alkaline phosphatase levels ranged from 125 to 243 units/liter and persisted for seven days to 48 months. Thus, biochemical evidence of mild cholestatic liver injury was common in patients receiving cyclosporine A. These abnormalities are usually self-limited and asymptomatic but may cause diagnostic difficulty if a preexisting liver disease is present.
Similar content being viewed by others
References
Busuttil RW, Goldstein LI, Danovitch GM, Ament ME, Memsic LBF: Liver transplantation today. Ann Intern Med 104:377–389, 1986
Scharschmidt BF: Human liver transplantation: Analysis of data on 540 patients from four centers. Hepatology 4:95S-101S, 1984
Klintmalm GBG, Iwatsuki S, Starzl TE: Cyclosporin A hepatotoxicity in 66 renal allograft recipients. Transplantation 32:488–489, 1981
Atkinson K, Biggs J, Dodds A, Concannon A: Cyclosporin-associated hepatotoxicity after allogeneic marrow transplantation in man: Differentiation from other causes of post transplant liver disease. Transplant Proc 15:s2761–2767, 1983
McKenzie FN, Moses GC, Henderson AR: Routine “cardiac” and “hepatic” serum enzyme profiles in cardiac transplant patients treated with cyclosporine A: Operative and postoperative findings. Clin Chem 31:822–825, 1985
Laupacis A, Keown PA, Ulan RA, Sinclair NR, Stiller CR: Hyperbilirubinemia and cyclosporin A levels. Lancet 2:1426–1427, 1981
Schade RR, Guglielmi A, Van Thiel DH, Thompson ME, Warty V, Griffith B, Sanghvi A, Bahnson H, Hardesty R: Cholestasis in heart transplant recipients treated with cyclosporine. Transplant Proc 15:2757–2760, 1983
Loertscher R, Wenk M, Harder F, Brunner F, Follath F, Thiel G: Hyperbilirubinemia and cyclosporin A levels in renal transplant patients. Lancet 2:635–636, 1981
Lorber I, Van Buren CT, Flechner SM, Williams C, Kahan BD: Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients. Transplantation 43:35–40, 1987
Kahan BD, Flechner SM, Lorber MI, Golden D, Conley S, Van Buren CT: Complications of cyclosporine-prednisone immunosuppression in 402 renal allograft recipients exclusively followed at a single center from one to five years. Transplantation 43:197–204, 1987
Ferguson RM, Rynasiewicz JJ, Sutherland DER, Simmons RL, Najarian JS: Cyclosporin A in renal transplantation: A prospective randomized trial. Surgery 92:175–182, 1982
Palestine A, Nussenblatt R, Chan CC: Side effects of systemic cyclosporine in patients not undergoing transplantation. Am J Med 77:652–656, 1984
Stone BG, Udani M, Sanghvi A, Warty V, Plocki K, Dedetti CD, Van Thiel DH: Cyclosporin A-induced cholestasis. The mechanism in a rat model. Gastroenterology 93:344–351, 1987
Rotolo FS, Branum GD, Bowers BA, Meyers WC: Effect of cyclosporine on bile secretion in rats. Am J Surg 151:35–40, 1986
Nicchitta CV, Kamoun M, Williamson JR: Cyclosporin augments receptor-mediated cellular Ca fluxes in isolated hepatocytes. J Biol Chem 260:13613–13618, 1985
Stacey NH, Kotecka B: Inhibition of taurocholate and ouabain transport in isolated rat hepatocytes by cyclosporin A. Gastroenterology 95:780–786, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kassianides, C., Nussenblatt, R., Palestine, A.G. et al. Liver injury from cyclosporine A. Digest Dis Sci 35, 693–697 (1990). https://doi.org/10.1007/BF01540169
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01540169