Abstract
Induced hypothermia can be used to protect the brain from post-ischemic and traumatic neurological injury. Potential clinical applications and the available evidence are discussed in a separate paper. This review focuses on the practical aspects of cooling and physiological changes induced by hypothermia, as well as the potential side effects that may develop. These side effects can be serious and, if not properly dealt with, may negate some or all of hypothermia’s potential benefits. However, many of these side effects can be prevented or modified by high-quality intensive care treatment, which should include careful monitoring of fluid balance, tight control of metabolic aspects such as glucose and electrolyte levels, prevention of infectious complications and various other interventions. The speed and duration of cooling and rate of re-warming are key factors in determining whether hypothermia will be effective; however, the risk of side effects also increases with longer duration. Realizing hypothermia’s full therapeutic potential will therefore require meticulous attention to the prevention and/or early treatment of side effects, as well as a basic knowledge and understanding of the underlying physiological and pathophysiological mechanisms. These and other, related issues are dealt with in this review.
Similar content being viewed by others
Introduction
Induced hypothermia is being used with increasing frequency to provide protection for the brain, spinal cord and perhaps other organs, such as the heart, against post-ischemic and post-traumatic injury. A large body of evidence from animal experiments suggests that hypothermia may be effective in various clinical situations if applied appropriately and quickly enough. These observations have been confirmed by an increasing number of clinical studies showing that hypothermia can be successfully used clinically for indications such as post-hypoxic injury following cardiopulmonary resuscitation (CPR). These issues and the evidence supporting various clinical applications of induced hypothermia are discussed in a separate review.
The expanding use of hypothermia in medicine means that most intensivists and others working in the ICU are likely to be confronted with patients who are treated with artificial cooling. Therefore, it is important that those employing hypothermia as a medical tool obtain a basic understanding of the underlying mechanisms, the physiology of temperature regulation and the many physiological changes taking place when a patient is cooled. Moreover, hypothermia can be a two-edged sword; although significant benefits can be achieved, there are many potential side effects that, if left untreated, can diminish or even negate the potential benefits. These side effects, as well as physiological changes associated with cooling and various practical aspects in inducing hypothermia, are the topic of this review.
Physiology and mechanisms
Physiology of temperature regulation and induction of hypothermia
The human body can be roughly divided into two thermal compartments: a “core” compartment, consisting of the trunk and head, excluding the skin, and a “peripheral” compartment, consisting of the skin and extremities. Under normal circumstances core body temperature is strictly regulated around a set point of 36.60±0.38°C. Slight variations in this set point occur in the course of a day; usually body temperature is highest at ±18:00 h. The temperature of the peripheral compartment is less strictly controlled and, under normal circumstances, is 2–4°C lower than the core temperature. This difference increases in cold environments and decreases in warm environments. The core temperature is regulated by limiting or increasing heat transfer to the periphery through vasodilation or vasoconstriction; in turn; heat loss from the peripheral compartment is regulated through changes in skin perfusion (again through vasodilation or vasoconstriction) and by increasing or decreasing the production of sweat. When warm blood flows from the core to the periphery, heat is transferred from the blood to the surrounding tissues and to the cooler tissue near the skin. The rate of conduction from peripheral blood vessels to the outside depends on the diffusion coefficient, which is determined by tissue characteristics. For example, fat insulates about three times as well as muscle, so that obese patients will lose heat more slowly than those who are lean [1, 2]. In experiments in healthy volunteers, the increase in metabolic rate due to shivering is attenuated by the square root of percent body fat [2]. In addition, there are differences between different muscle groups in regard to the intensity of shivering and the amount of heat that can be generated. Muscles of the trunk region began to shiver sooner, and at a higher intensity, than those of the limbs [2].
Apart from sweat production (evaporation), heat loss can occur via convection, conduction and radiation (Table 1). The amount of heat loss depends on the temperature gradient, exposed surface and thermal conductivity. At rest and under normal circumstances 50–70% of the heat loss in awake patients occurs through radiation [1, 3]. In sedated patients in the ICU most heat loss will occur via radiation and convection. When patients are actively cooled this is often accomplished by facilitating convection and/or conduction, as well as by facilitating the transfer of heat from the core to the peripheral compartment (see below).
Induction of hypothermia
If hypothermia develops (either accidentally or intentionally induced), the body will immediately try to counteract this disturbance in homeostasis. The initial response will be to decrease heat loss, mainly through increasing sympathetic tone and through vasoconstriction in the skin. This response complicates attempts to induce therapeutic hypothermia by external cooling (see below). In addition, heat production will be increased through shivering and, in later phases, through the increased metabolism of fats, carbohydrates and proteins. Shivering can lead to increases in oxygen consumption of between 40% and 100% [4, 5, 6], an undesirable effect particularly in patients with neurological and/or posthypoxic injury. These responses can be counteracted by the administration of sedatives, anesthetics, opiates and/or paralyzing drugs (see below). Sedation and anesthesia also increase peripheral blood flow, thereby increasing the transfer of heat from the core to the periphery. As explained above, the rate of heat loss is determined by the temperature gradient, body composition and the conductive properties of the environment. For example, water is a much better conductor of heat than air and, thus, wet skin will transfer heat much more easily than dry skin. Heat loss is further increased by the use of alcohol-based, rather than water-based, solutions.
It should be noted that the capacity and effectiveness of the mechanisms to control body temperature decrease with age. Younger patients will therefore react earlier and with greater intensity and effectiveness to changes in body temperature than older patients. In addition, older patients have a lower rate of metabolism, often a lower body mass index (BMI) and less effective vascular response (i.e., less vasoconstriction). Thus, in general, the induction of hypothermia in younger patients will be significantly more difficult than in older patients. Induction of hypothermia in younger patients often requires high doses of sedatives to counteract the above-mentioned counter-regulatory mechanisms. Similarly, achieving hypothermia through surface cooling in obese patients will take more time due to the insulating properties of fat. This implies that the surface cooling of obese patients will be more difficult and require significantly more time to achieve target temperatures.
Metabolic and cellular effects of hypothermia
Hypothermia affects many intracellular processes. Some of these are directly related to its protective effects; these aspects are discussed in more detail in Part 1 of this review. Here we will focus on those features that are relevant to physiological and pathophysiological changes induced by cooling. These changes are listed in Tables 2, 3 and 4.
Hypothermia leads to a lowering of the metabolic rate. Indeed, in the past it was assumed that the protective effects of hypothermia were due solely to the slowing of cerebral metabolism, with associated decreases in consumption of glucose and oxygen. It has since become clear that other mechanisms are involved, which probably play a much greater role than the changes in metabolic rate. These issues are discussed in Part 1 of this review. Nevertheless, the effects on metabolism are significant and probably do play a part in providing neuroprotection. In addition, these changes in metabolism occur in all organ systems; this means, for example, that there will be a decrease in oxygen consumption and carbon dioxide production (which implies that ventilator settings should be adjusted), a reduction in feeding requirements, etc. Metabolism is reduced by between 5% and 7% per Celsius degree reduction in body temperature [7, 8, 9]. Cerebral blood flow is also decreased, but, when corrected for the decrease in metabolism, the net result is a relative increase.
Many hypothermia-induced metabolic changes occur relatively quickly, within the first few hours. These include changes in energy metabolism and decreases in adenosine tri-phosphate (ATP) demand. Other changes, such as a rise in lactate levels, occur over a longer period of time (>3 h). Induction of hypothermia also leads to an increase in membrane stability, with decreased permeability of cellular membranes, the blood-brain barrier and blood vessel walls [10, 11, 12, 13, 14]. One of the consequences of this is a decrease in edema formation, that appears to be one of the ways in which hypothermia can protect against neurological injury. In addition, hypothermia can prevent or mitigate the excessive influx of Ca2+ into the cell, as well as decrease accumulation of the excitatory neurotransmitter glutamate in the extracellular space [15]. Calcium influx and glutamate accumulation are key elements in the destructive cascade that can follow a period of ischemia; calcium influx into the cell can lead to mitochondrial dysfunction and the activation of various enzymes which can cause additional cell injury and death [15]. Hypothermia also leads to a decrease in intracellular acidosis (although the extracellular pH usually decreases slightly during cooling, due to increased levels of lactic acid, glycerol, free fatty acids and ketonic acids; see below).
Hypothermia also influences the immune system, with an inhibition of neutrophil and macrophage function, suppression of inflammatory reactions and inhibition of the release of pro-inflammatory cytokines [16, 17, 18]. This effect on immune response may contribute to hypothermia’s neuroprotective effects, but, of course, increases the risk of infections (see below). Other anti-inflammatory mechanisms include the prevention or mitigation of reperfusion-related DNA injury, lipid peroxidation and leukotriene production as well as a decrease in the production of nitric oxide [19, 20]. In addition, hypothermia decreases reperfusion injury and free radical production [19].
Practical aspects and side effects
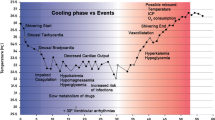
Induction of hypothermia causes a large number of physiological changes in the circulatory and respiratory systems, coagulation system, drug metabolism, etc. (listed in Table 2). For the successful use of hypothermia, awareness of these physiological effects and pathophysiological mechanisms is of key importance. The failure to demonstrate positive effects of hypothermia in some clinical trials may be partly due to insufficient regard for side effects causing the (partial) negation of protective effects. In addition, unawareness of hypothermia’s physiological consequences may lead to over-treatment. For example, even mild hypothermia induces decreases in cardiac output, mild acidosis, a rise in lactate levels and a moderate increase in levels of amylase. These changes are normal, do not signify any deterioration in the patients’ condition and do not require treatment. Naturally, such changes can sometimes be unwanted, such as shivering with its associated rise in oxygen consumption and patient discomfort. Many of these physiological effects can be counteracted by appropriate medication, such as sedatives, analgesics or paralyzers. The use of therapeutic hypothermia will usually require ICU admission and monitoring and often (but not always) sedation and intubation.
The physiological and pathophysiological effects of cooling largely depend on the degree of hypothermia. For example, a significant risk for severe arrhythmias occurs only at temperatures below 28–30°C. Such low temperatures are now rarely employed in induced hypothermia, although they are used more frequently in specific surgical procedures, such as major vascular surgery. This review will focus on the effects of mild to moderate hypothermia (31–35°C).
The physiological adaptations to hypothermia, changes in laboratory values and potential side effects are listed in Tables 2, 3 and 4. These changes depend to varying degrees on the patients’ age, underlying disease, co-morbidity etc. Some of these changes can be suppressed or prevented by medication, appropriate sedation or other factors.
Cardiovascular and hemodynamic effects
Hypothermia is initially associated with sinus tachycardia, after which bradycardia develops. This is partly due to decreases in metabolism and partly to the direct effects of hypothermia on the heart. Various ECG changes may occur (listed in Table 2). The risk of arrhythmias during mild or moderate hypothermia is very low, but increases significantly when the temperature drops below 30°C. The initial arrhythmia is usually atrial fibrillation, which can be followed (at temperatures ≤28°C) by the risk of ventricular flutter or fibrillation. An additional problem is that arrhythmias in deeply hypothermic patients are difficult to treat, as the myocardium becomes less responsive to defibrillation and anti-arrhythmic drugs. When therapeutic hypothermia is applied, therefore, great care should be taken to keep temperatures at 30°C or more, as the risk of clinically significant arrhythmias increases exponentially below this temperature level.
Initially, the induction of mild hypothermia increases myocardial oxygen demand relative to supply; the mechanism is probably a hypothermia-induced increase in plasma levels of adrenaline and noradrenaline leading to an increase in cardiac output and oxygen demand [21]. With further reductions in temperature, decreases in heart rate and the slowing of metabolism will reduce cardiac afterload and oxygen demand. Mild hypothermia decreases cardiac output by about 25% and leads to increased vascular resistance and a rise in central venous pressure. During severe hypothermia (≤30°C) left ventricular contractility itself may decrease, inducing systolic and diastolic dysfunction. In healthy subjects mild hypothermia (35.5°C) has been shown to increase coronary perfusion [21, 22]. However, one study reported that, in patients with pre-existent coronary artery disease, coronary vasoconstriction may occur during hypothermia [22]. This difference is presumed to be caused by endothelial dysfunction associated with atherosclerosis [21]. This would imply that there is a theoretical risk of myocardial injury during the induction of mild hypothermia in patients with cardiovascular disease, especially in the phase when cooling is initiated and the heart rate temporarily increases.
On the other hand, there is strong evidence from animal studies that the induction of hypothermia during or following myocardial infarction can decrease the infarct size [23, 24, 25, 26, 27, 28, 29, 30]. Hypothermia has been used in one clinical study in 42 patients with acute myocardial infarction undergoing emergency percutaneous coronary intervention [31]. Twenty-one patients were treated with hypothermia for 3 h after reperfusion, the other patients served as controls. The hypothermia group had a trend to smaller infarct sizes and fewer major adverse cardiac events, though these differences did not reach statistical significance in this small number of patients. Although firm conclusions regarding the benefits for cases of myocardial infarctions cannot yet be drawn, these data do at least suggest that hypothermia did not adversely effect outcome in these patients with coronary artery disease.
Coagulation
Hypothermia induces a mild bleeding diathesis, with increased bleeding time due to its effect on platelet count [32, 33], platelet function [32, 33, 34], the kinetics of clotting enzymes and plasminogen activator inhibitors [35, 36] and other steps in the coagulation cascade [36, 37, 38]. It should be pointed out that the laboratory results of standard coagulation tests such as prothrombin time and partial thromboplastin times will remain normal, because these tests are usually performed at 37°C in the lab. Tests will be prolonged only if they are performed at the patient’s actual core temperature [39]. However, in spite of the above-mentioned abnormalities, the risk of significant bleeding is very low, even in patients with traumatic brain injury (TBI) [40]. None of the clinical trials in patients with TBI, subarachnoid hemorrhage, stroke or post-anoxic coma have reported increased intracranial bleeding associated with cooling. These observations are confirmed by data from animal experiments showing decreased extravasation of hemoglobin during hypothermia [12]. Overall, few bleeding complications were seen in any of the major clinical trials using hypothermia, and risks of bleeding should therefore not preclude the use of hypothermia if deemed appropriate. Platelets and/or fresh frozen plasma can be administered to improve coagulation if necessary.
Coagulation disorders may be a greater problem in trauma patients. Here the use of therapeutic hypothermia is somewhat controversial and a potential conflict between ‘protecting the brain and protecting the body’ may arise. Various studies have reported an association between hypothermia and adverse outcome in trauma patients [41, 42, 43]; this link has given hypothermia an ominous reputation among trauma surgeons and has led to recommendations of the aggressive re-warming of trauma patients. However, its should be pointed out that most of these studies were uncontrolled and retrospective, and in most cases no multivariate analysis was performed to correct for potential confounders [review of this issue: 44]. One study that did perform multivariate analysis, correcting for factors such as presence of shock (associated with both hypothermia and adverse outcome, and therefore a potential confounder) concluded that hypothermia is a marker, but not a cause, of adverse outcome [45]. Thus, although hypothermia does induce a degree of coagulopathy, its reputation in trauma patients may be partly undeserved; the use of therapeutic hypothermia in trauma patients should, therefore, not be automatically excluded. This view is underscored by observations that active re-warming of hypothermic patents with TBI may adversely affect outcome [46]. We therefore recommend that the use of hypothermia be considered in trauma patients who meet inclusion criteria as set out in Part 1 of this review (for example, patients who have undergone CPR with unclear neurological outcome) provided they do not have active bleeding and are hemodynamically stable.
Infection
Evidence from clinical and in vitro studies shows that hypothermia can impair immune function. Indeed, (as discussed above) inhibition of inflammatory responses may be one of the mechanisms through which hypothermia exerts neuroprotective effects. Hypothermia inhibits the release of various pro-inflammatory cytokines [16, 17] and suppresses chemotactic migration of leukocytes and phagocytosis [47]. Hypothermia-induced insulin resistance and hyperglycemia may further increase infection risks (see below). Thus, there are plausible mechanisms for an immunosuppressive effect of hypothermia.
A number of studies, mostly in patients with stroke or TBI, have indeed reported higher risks of pneumonia when therapeutic hypothermia is used over longer periods of time (≥48–72 h) [48, 49]. However, other studies using hypothermia for prolonged periods in patients with TBI reported no increase in infection rates [50, 51]. This may be attributable to antibiotic prophylaxis or selective decontamination of the digestive tract (SDD), which were used in some of these studies [51]. Short-term cooling (≤24 h) does not appear to increase the risk of infection [50, 52, 53]. Overall, the risk of respiratory tract infections appears to increase when patients are cooled for 48 h or more; this problem appears manageable with rigorous surveillance and, perhaps, prophylactic measures.
Some studies have also reported a higher risk of wound infections associated with hypothermia [54, 55]. This may be related to both diminished leukocyte function and hypothermia-induced vasoconstriction. Animal studies have shown that the establishment of infection probably occurs within the first 3 h of bacterial inoculation [55, 56, 57] and is facilitated by local vasoconstriction and hypoperfusion. This may be important in patients requiring surgery during treatment with hypothermia. Moreover, other wounds, including bed sores and catheter insertion sites, are more likely to show progression and/or impaired healing during cooling.
Hypovolemia, fluid balance and electrolytes
The induction of hypothermia can lead to the loss of significant amounts of fluids, due to so-called hypothermia-induced diuresis [58, 59, 60, 61]. This may be especially pronounced in patients with TBI, in whom diabetes insipidus, induced by cranial trauma, and administration of medication such as mannitol may exacerbate fluid losses [51, 59, 60]. The impact of this may be significant, especially in patients with TBI or subarachnoidal hemorrhage (SAH) where even very brief episodes of hypovolemia or hypotension can significantly, and adversely, affect outcome [62, 63, 64]. Indeed, any beneficial effects of hypothermia may be lost due to side effects if these are not treated proactively and vigorously [58]. Therefore, close attention should be paid to the patients’ diuresis and fluid balance especially during induction of hypothermia (i.e., the phase when the patients’ body temperature is decreasing, which is the phase when excessive diuresis and hypovolemia are most likely to occur [51, 58, 59]). In our center we infuse 500–1000 ml of saline and electrolytes (see below) in TBI patients upon initiation of cooling (provided the patients are young and have no significant counter-indications) and supplement fluid losses that occur during cooling [51, 58, 59]. However, these problems are much less evident in other categories of patients, such as those with post-anoxic coma following CPR [52, 53, 65]. The reason for this difference is probably that the risk of excessive fluid loss in TBI patients is caused by a combination of hypothermia and other factors, such as the administration of mannitol to decrease intracranial pressure.
Another important problem is induction of electrolyte disorders. We and others have observed severe electrolyte disorders (i.e., low levels of Mg, K, P and Ca) during cooling of patients with TBI [59, 66]. Such electrolyte disorders can cause cardiac arrhythmias as well as hypotensive episodes with decreases in cerebral blood flow. Magnesium may be especially important in this regard, because of its specific role in mitigating neurological injuries [67, 68, 69, 70, 71]. Intracellular free magnesium in the brain declines by up to 60% following moderate traumatic brain injury in rats [72]. Numerous animal studies have shown that magnesium depletion leads to significantly worse outcomes in experimental TBI; administration of magnesium before or even after trauma substantially mitigates secondary injury and reduces the loss of cortical cells [67, 68, 69, 73, 74, 75, 76]. Magnesium may also play a role in the prevention of reperfusion injury, which is one of the key mechanisms underlying secondary neurological injury [76]. In addition, loss of magnesium is associated with vasoconstriction of cerebral and coronary arteries [77, 78, 79].
Clinical studies in ICU patients have shown that hypomagnesemia is associated with adverse outcome [80]. Severe head injury itself is associated with significant loss of electrolytes including magnesium [71]; thus many patients with TBI have hypomagnesemia at admission [71], which subsequently can be significantly exacerbated by the induction of hypothermia [59]. Electrolyte disorders are easily treated or prevented; physicians utilizing induced hypothermia should be aware of these risks. It should be noted that serum levels do not always accurately reflect magnesium status [79], and in our opinion magnesium levels should be maintained in the high or high-normal range in all patients with neurological injury [70]. Other electrolytes, such as phosphorus and potassium, should also be monitored closely and maintained in the high-normal range [70].
Other metabolic effects
Hypothermia decreases insulin sensitivity and insulin secretion, which can lead to hyperglycemia. Hyperglycemia is associated with increased infection rates, increased incidence of renal failure and critical illness neuropathy and various other complications, while prevention of hyperglycemia and tight control of glucose levels may decrease morbidity and mortality in ICU patients [81]. These protective effects are due to the prevention of hyperglycemia per se rather than to the direct effects of insulin [82, 83]. This underscores the importance of tight glucose regulation, especially in patients treated with hypothermia in whom hyperglycemia is more likely to develop. The amounts of insulin required to maintain glucose levels within the normal range are likely to increase during the induction of hypothermia, and physicians applying hypothermia should be aware of this phenomenon.
Hypothermia also induces mild acidosis through various mechanisms including increased synthesis of glycerol, free fatty acids, ketonic acids and lactate. These changes are normal metabolic consequences of hypothermia and should not be attributed to complications such as bowel ischemia.
Shivering
As discussed in “Physiology and mechanisms”, the body will employ various mechanisms to generate heat, including shivering which may increase oxygen consumption and patient discomfort. In ventilated patients this can be counteracted by the administration of sedatives and analgesics or, if deemed appropriate, the administration of muscle paralyzers. Shivering can be attenuated by relatively small doses of opiates; meperidine (pethidine) appears to be somewhat more effective in this regard due to a higher activity at the kappa receptor [15]. This means that lower doses (12.5–25 mg) can be used, which may be especially important if hypothermia is used in awake patients. When using paralyzers and/or opiates is deemed undesirable, alternatives with which to treat shivering include the administration of clonidine, neostigmine and ketanserine. However, care should be taken to avoid adverse effects; for example, clonidine may aggravate hypothermia-induced bradycardia.
Miscellaneous
Another important issue is the effect of hypothermia on drug metabolism and pharmacokinetics. The enzymes that metabolize most drugs are highly temperature-sensitive and, thus, drug metabolism is significantly affected by hypothermia. Clearance of various drugs is decreased and in most patients doses should be lowered during hypothermia. Unfortunately, few data are available regarding the effects of hypothermia on the metabolism of specific drugs. However, the studies that have been carried out confirm the expectation that plasma levels increase and the effects of drugs are prolonged. For example, plasma levels of propofol increase by approximately 30% and of fentanyl by 15% when individuals are 3°C hypothermic [55]. A number of the medications for which data are available are listed in Table 2.
Cooling methods and practical guidelines
Methods to induce hypothermia
There are numerous strategies to cool patients, based on the four basic mechanisms for heat loss: convection, conduction, evaporation and radiation. In addition, heat generation in patients with hyperthermia can sometimes be reduced by antipyretic agents. However, in patients with elevated temperature caused by impaired thermoregulation (such as central fever or heat stroke) these agents are often ineffective. Various cooling techniques have been used in in vitro and clinical studies, including ice-water circulating blankets, ice bags, air mattresses, cooling catheters, intravenous infusion of cooled fluids (4°C) followed by cooling through other methods, the infusion of extracorporeally cooled blood via the carotid artery, helmets and cooling caps with cold fluids or chemical cooling capabilities, ice-water nasal lavage, cold peritoneal lavage and cardiopulmonary bypass [31, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93]. The methods most commonly employed in the clinical setting are summarized in Table 5. Cooling caps and coils wrapped around the head have been used mainly in infants and neonates, but have also been tried in adults.
Most large clinical trials published so far have used either water-cooling or air-cooling blankets. Air-cooling blankets have also been used in general wards in awake patients [94, 95]. Water-cooling blankets are much more efficient for cooling than for warming patients, because the temperature difference can be set much higher; during warming a set temperature above 40–42°C can cause burns, whereas the skin is much more tolerant of lower temperatures. As explained in “Physiology and mechanisms”, the speed of inducing hypothermia may be important in achieving optimum effects. The times required to achieve target temperatures have varied considerably in the clinical trials published so far, ranging from approximately 2 h [51, 52] to around 8 h [46, 53] or even longer [49]. These time periods depend on patient factors (nature of the underlying disease or injury, age, sex, BMI), countermeasures to prevent shivering and heat generation, and on technical aspects such as the cooled surface, temperature of the blankets or air, cooling capacity, etc. In a recently published study in patients with TBI we were able to induce temperatures of 34°C or below in 95% of our patients within 2 h, by using two cooling blankets (above and below the patient) with the water temperature set at 4°C until the core temperature was 33°C or less (the target temperature being 32°C), and by using water and alcohol sprays and exposing the areas of skin that were not directly cooled [51]. Heat transfer from core to periphery may also be facilitated by the use of vasodilatory medication.
An even quicker method was described in a preliminary report by Bernard et al. [96], who used large volumes (30 ml/kg) of ice-cold (4°C) intravenous fluid (lactated Ringer’s solution) to cool 22 comatose survivors of out-of-hospital cardiac arrest quickly. These authors were able to decrease core temperature from 35.5 to 33.8°C within 30 min with no adverse consequences, and concluded that this was an inexpensive and effective method of initially inducing mild hypothermia. We have used this method in selected cases in our own clinic with good results and no adverse effects.
A new development is the availability of intravascular cooling catheters such as the CoolLine, SetPoint and others (Table 5). These are central lines with two or three balloons filled with temperature-controlled saline, allowing direct intravascular (and, therefore, core) cooling via the subclavian, superior caval or femoral vein. Experience with these catheters has been relatively limited so far, although they are rapidly gaining in popularity. Initially, two small feasibility trials in six [90] and eight patients [91] reported that it was an effective, relatively quick method to induce and maintain hypothermia, and that it was less labor-intensive than the ‘conventional methods’ listed above. Of note, no thrombus formation on these catheters was observed upon removal in these studies. Endovascular cooling has also been used to induce brief periods of hypothermia in 20 non-sedated patients undergoing percutaneous coronary intervention with the aim of reducing infarct size [31]. A larger trial using this device to cool 51 patients with hyperthermia in a neurosurgical ICU has recently been published; the authors reported that the device was safe and effective in inducing hypothermia [92]. However, no studies have yet been published in which these devices have been used for longer-term (>24 h) cooling.
Yet more novel approaches include the use of selective brain cooling [86], peritoneal cooling [88] and ice-water nose cooling [93]. Experience with these methods is limited to animal studies and/or small case series [93]. Treatment with paracetamol or acetaminophen may serve as an accessory method to lower temperature, especially in patients with hyperthermia, although their effectiveness in patients with neurological injury is limited [94].
Practical guidelines
Therapeutic hypothermia can be used in various types of neurological injury and perhaps for other indications, such as the prevention of reperfusion injury. The use of hypothermia will often require intubation, mechanical ventilation, sedation and, at times, pharmacologic paralysis to prevent shivering. A major problem induced by these measures is that they may significantly hamper neurological assessment of the patient; thus patients should be carefully monitored for, for example, the development of seizures, which may present much less clearly. As outlined in “Practical aspects and side effects”, the induction of hypothermia can cause a number of side effects; however, many of these can be prevented or attenuated. Fluid balance should be carefully monitored and hypovolemia and hypotension avoided, especially in patients with TBI or SAH. Electrolyte disorders (especially hypomagnesemia) and arrhythmias should be prevented, if necessary by the early or prophylactic administration of anti-arrhythmic agents. This also applies to hyperglycemia, which should be combated through intensive insulin therapy and frequent monitoring.
Infections should be prevented by early or even prophylactic treatment with antibiotics. Bleeding complications can be avoided by the timely administration of platelets or fresh frozen plasma, particularly if surgical interventions or invasive procedures are performed. From this perspective, realizing hypothermia’s full therapeutic potential presents a great challenge to ICU physicians, requiring first-rate quality in many aspects of intensive care. This applies not only to intensivists but, in equal measure, to ICU nurses and others caring for critically ill patients in the ICU. Hypothermia-induced vasoconstriction of the skin and increased risk of wound infections will increase the risk of bed sores, as will the sedation and paralysis often required in these patients. The risk of respiratory tract infections may increase, requiring extra vigilance and interventions by the nursing staff, physiotherapist and others. Infections should be treated promptly and aggressively. The use of selective decontamination of the digestive tract should be considered in these patients.
The insertion sites of central venous catheters will require close monitoring and extra care. The patient may become relatively unstable, especially in the cooling phase while the core temperature is decreasing. Polyuresis, electrolyte disorders and hypotension may develop, especially in patients with TBI; ventilator settings will need to be changed as oxygen demand and production of carbon dioxide decrease due to the slowing of metabolism. Blood samples must be frequently drawn and analyzed on site or sent for analysis; the patient must be closely monitored for any signs of shivering, seizures etc. All this will, at least temporarily, increase the nursing work load. This is something to keep in mind as various studies have demonstrated that shortages of nursing and medical staff due to increased workload can adversely affect outcome [97].
The successful application of hypothermia thus requires a concerted team effort and the difficulties involved, as well as potential side effects and risks, should not be underestimated. Using this therapy requires vigilance, attentiveness and experience. ICUs considering the use of therapeutic hypothermia should adopt strict guidelines and protocols, and provide training for ICU physicians, nursing staff and other members of the ICU team. When hypothermia has been used it is important that patients should not be re-warmed too quickly, as this may have adverse effects especially in patients with TBI.
Summary and conclusions
A large body of evidence suggests that hypothermia can be used to prevent or limit damage to the injured brain and spinal cord, and perhaps the heart, in selected categories of patients. It is important to induce hypothermia as quickly as possible, as protection appears to be greater when cooling is initiated early (although benefits have been reported even when cooling was initiated many hours after injury). As shown in this review, the induction of hypothermia will affect every organ in the body and it is important that ICU staff members are aware of this and are able to distinguish physiological changes from pathophysiological side effects.
The successful application of hypothermia requires the use of strict protocols, vigilance by the medical and nursing staff, and close attention to the prevention of side effects. The volume status of patients treated with cooling should be monitored carefully, to prevent hypovolemia and hypotension. Other measures should include the frequent monitoring of electrolyte levels to avoid electrolyte disorders (especially hypomagnesemia) and prevention or early treatment of arrhythmias, if necessary by early or prophylactic administration of anti-arrhythmic agents. In addition, hyperglycemia should be avoided through intensive insulin therapy and frequent monitoring of glucose levels, and infections should be prevented by early or prophylactic treatment. Bleeding complications can be avoided by the administration of platelets or plasma if surgical interventions or invasive procedures are required. Patients should be re-warmed slowly, as too rapid re-warming can adversely affect outcome.
References
Sessler DI (2000) Perioperative heat balance. Anesthesiology 92:578–594
Tikuisis P, Bell DG, Jacobs I (1991) Shivering onset, metabolic response and convective heat transfer during cold air exposure. J Appl Physiol 70:1996–2002
English MJM (2001) Physical principles of heat transfer. Curr Anaesth Crit Care 12:66–71. DOI 10.1054/cacc.2001.0331
Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, Cheng C (1995) Heat flow and distribution during induction of general anesthesia. Anesthesiology 82:662–673
Frank SM, Fleisher LA, Olson KF, Gorman RB, Higgins MS, Breslow MJ, Sitzmann JV, Beattie C (1995) Multivariate determinates of early postoperative oxygen consumption: the effects of shivering, core temperature and gender. Anesthesiology 83:241–249
Horvath SM, Spurr GB, Hutt BK, Hamilton LH (1956) Metabolic cost of shivering. J Appl Physiol 8:595–602
Milde LN (1992) Clinical use of mild hypothermia for brain protection: a dream revisited. J Neurosurg Anesthesiol 4:211–215
Small DL, Morley P, Buchan AM (1999). Biology of ischemic cerebral cell death. Prog Cardiovasc Dis 42:185–207
Schaller B, Graf R (2003) Hypothermia and stroke: the pathophysiological background. Pathophysiology 10:7–35
Fischer S, Renz D, Wiesnet M, Schaper W, Karliczek GF (1999) Hypothermia abolishes hypoxia-induced hyperpermeability in brain microvessel endothelial cells. Brain Res Mol Brain Res 74:135–144
Chopp M, Knight R, Tidwell CD, Helpern JA, Brown E, Welch KM (1989) The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab 9:141–148
Kinoshita K, Chatzipanteli K, Alonso OF, Howard M, Dietrich WD (2002) The effect of brain temperature on hemoglobin extravasation after traumatic brain injury. J Neurosurg 97:945–953
Chi OZ, Liu X, Weiss HR (2001) Effects of mild hypothermia on blood-brain barrier disruption during isoflurane or pentobarbital anesthesia. Anesthesiology 95:933–938
Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM (1999) Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci 26:298–304
Siesjo BK, Bengtsson F, Grampp W, Theander S (1989) Calcium, excitotoxins and neuronal death in brain. Ann NY Acad Sci 568:234–251
Kimura A, Sakurada S, Ohkuni H, Todome Y, Kurata K (2002) Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit Care Med 30:1499–1502
Aibiki M, Maekawa S, Ogura S, Kinoshita Y, Kawai N, Yokono S (1999) Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotraum 16:225–232
Frank SM (2001) Consequences of hypothermia. Curr Anaesth Crit Care 12:79–86
Busto R, Globus MY, Dietrich WD, Martinez E, Valdés I, Ginsberg MD (1989) Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 20:904–910
Dempsey RJ, Combs DJ, Maley ME, Cowen DE, Roy MW, Donaldson DL (1987) Moderate hypothermia reduces postischemic edema development and leukotriene production. Neurosurgery 21:177–181
Frank SM, Satitpunwaycha P, Bruce SR, Herscovitch P, Goldstein DS (2003) Increased myocardial perfusion and sympathoadrenal activation during mild core hypothermia in awake humans. Clin Sci (Lond) 104:503–508
Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP (1988) Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 77:43–52
Hale SL, Dae MW, Kloner RA (2003) Marked reduction in no-reflow with late initiation of hypothermia in a rabbit myocardial infarct model. J Am Coll Cardiol 41 (6 Suppl B):381–382
Hale SL, Dae MW, Kloner RA (2003) Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res 59:715–722
Hale SL, Kloner RA (2002) Elevated body temperature during myocardial ischemia/reperfusion exacerbates necrosis and worsens no-reflow. Coron Artery Dis 13:177–181
Hale SL, Kloner RA (1998) Myocardial temperature reduction attenuates necrosis after prolonged ischemia in rabbits. Cardiovasc Res 40:502–507
Miki T, Liu GS, Cohen MV, Downey JM (1998) Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol 93:372–383
Hale SL, Dave RH, Kloner RA (1997) Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol 92:351–357
Hale SL, Kloner RA (1997) Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol 273(1 Pt 2):H220–227
Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA (2002) Effect of endovascular cooling on myocardial temperature, infarct size and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol 282:H1584–1591
Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, Jenkins JS, Baim DS, Gibbons RJ, Kuntz RE, Popma JJ, Nguyen TT, O’Neill WW (2002) Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol 40:1928–1934
Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD (1987) Hypothermia-induced reversible platelet dysfunction. Ann Surg 205:175–181
Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR (1994) Hypothermia-induced reversible platelet dysfunction. Thromb Haemost 71:633–640
Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C (1998) Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function and fibrinolytic activity. J Trauma 44:846–854
Valeri CR, MacGregor H, Cassidy G, Tinney R, Pompei F (1995) Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med 23:698–704
Patt A, McCroskey B, Moore E (1988) Hypothermia-induced coagulopathies in trauma (Review). Surg Clin North Am 68:775–785
Ferrara A, MacArthur JD, Wright HK, Modlin IM, McMillen MA (1990) Hypothermia and acidosis worsen coagulopathy in the patients requiring massive transfusion. Am J Surg 160:515–518
Reed RL, Bracey AW, Hudson JD, Miller TA, Fischer RP (1990) Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock 32:141–152
Rohrer MJ, Natale AM (1992) Effect of hypothermia on the coagulation cascade. Crit Care Med 20:1402–1405
Resnick DK, Marion DW, Darby JM (1994) The effect of hypothermia on the incidence of delayed traumatic intracerebral hemorrhage. Neurosurgery 34:352–356
Jurkovich GJ, Greiser WB, Luterman A, Curreri PW (1987) Hypothermia in trauma victims: an ominous predictor of survival. J Trauma 27:1019–1024
Luna GK, Maier RV, Pavlin EG, Anardi D, Copass MK, Oreskovich MR (1987) Incidence and effect of hypothermia in seriously injured patients. J Trauma 27:1014–1018
Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O’Keefe GE (1997) Is hypothermia in the victim of major trauma protective or harmful? Ann Surg 226:439–447
Tisherman SA, Rodriguez A, Safar P (1999) Trauma care in the new millennium. Therapeutic hypothermia in traumatology. Surg Clin North Am 79:1269–1289
Steinemann S, Shackford SR, Davis JW (1990) Implications of admission hypothermia in trauma patients. J Trauma 30:200–202
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Muizelaar JP, Wagner FC, Marion DW, Luerssen TG, Chesnut RM, Schwartz M (2001) Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 344:556–563
Salman H, Bergman M, Bessler H, Alexandrova S, Beilin B, Djaldetti M (2000) Hypothermia affects the phagocytic activity of rat peritoneal macrophages. Acta Physiol Scand 168:431–436
Shiozaki T, Hayakata T, Taneda M, Nakajima Y, Hashiguchi N, Fujimi S, Nakamori Y, Tanaka H, Shimazu T, Sugimoto H (2001) A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg 94:50–54
Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA (2001) Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 32:2033–2035
Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST (1997) Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 336:540–546
Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes ARJ (2002) Effects of artificially induced hypothermia on intracranial pressure and outcome in patients with severe traumatic head injury. Intensive Care Med 28:1563-1567
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563
The Hypothermia after Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346:549–556
Kurz A, Sessler DI, Lenhardt R and the Study of Wound Infection and Temperature Group (1996) Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. New Engl J Med 334:1209–1215
Sessler DI (2001) Complications and treatment of mild hypothermia. Anesthesiology 95:531–543
Sheffield CW, Sessler DI, Hunt TK (1994) Mild hypothermia during isoflurane anesthesia decreases resistance to E. Coli dermal infection in guinea pigs. Acta Anesthesiol Scand 38:201–205
Sheffield CW, Sessler DI, Hunt TK, Scheuenstuhl H (1994) Mild hypothermia during halothane anesthesia decreases resistance to S. Aureus dermal infection in guinea pigs. Wound Rep Reg 2:48–56
Polderman KH, Girbes ARJ, Peerdeman SM, Vandertop WP (2001) Hypothermia (review/comment). J Neurosurgery 94:853–855
Polderman KH, Peerdeman SM, Girbes ARJ (2001) Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. Journal of Neurosurg 94:697–705
Kaufman HH, Timberlake G, Voelker J, Pait TG (1993) Medical complications of head injury. Med Clin North Am 77:43–60
Weinberg AD (1993) Hypothermia. Ann Emerg Med 22:370–377
The Brain Trauma Foundation. The American Association of Neurological Surgeons (2000) The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma 17:507–511
Fearnside MR, Cook RJ, McDougall P, McNeil RJ (1993) The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg 7:267–279
Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF (1993) Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 59:121–125
Polderman KH, Sterz F, van Zanten ARH, Uray T, Losert H, de Waal R, Girbes ARJ, Holzer M (2003) Induced hypothermia improves neurological outcome in asystolic patients with out-of hospital cardiac arrest (abstract). Circulation 108:IV-581 [abstract 2646]
Aibiki M, Kawaguchi S, Maekawa N (2001) Reversible hypophosphatemia during moderate hypothermia therapy for brain-injured patients. Crit Care Med 29:1726–1730
McIntosh TK Vink R, Yamakami I, Faden AI (1989) Magnesium protects against neurological deficit after brain injury. Brain Res 482:252–260
Vink R (1988) Decline in intracellular free Mg2+ is associated with irreversible tissue injury after brain trauma. J Biol Chem 263:757–761
Vink R, Cernak I (2000) Regulation of intracellular free magnesium in central nervous system injury. Front Biosci 5:656–665
Polderman KH, Zanten ARH van, Girbes ARJ (2003) The importance of magnesium in critically ill patients: a role in mitigating neurological injury and in the prevention of vasospasms. Intensive Care Med 29:1202–1203
Polderman KH, Bloemers F, Peerdeman SM, Girbes ARJ (2000) Hypomagnesemia and hypophosphatemia at admission in patients with severe head injury. Crit Care Med 28:2022–2025
Dietrich WD, Alonso O, Busto R, Globus MYT, Ginsberg MD (1994) Posttraumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol 87:250–258
Vacanti FX, Ames AA (1983) Mild hypothermia and Mg++ protect against irreversible damage during CNS ischemia. Stroke 15:695–698
Saatman KE, Bareyre FM, Grady MS, McIntosh TK (2001) Acute cytoskeletal alterations and cell death induced by experimental brain injury are attenuated by magnesium treatment and exacerbated by magnesium deficiency. J Neuropathol Exp Neurol 60:183–194
Bareyre FM, Saatman KE, Raghupathi R, McIntosh TH (2000) Postinjury treatment with magnesium chloride attenuates cortical damage after traumatic brain injury in rats. J. Neurotrauma 17:1029–1039
Garcia LA, Dejong SC, Martin SM, Smith RS, Buettner GR, Kerber RE (1998) Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J Am Coll Cardiol 32:536–539
Pyne GJ, Cadoux-Hudson TA, Clark JF (2001) Magnesium protection against in vitro cerebral vasospasm after subarachnoid haemorrhage. Br J Neurosurg 15:409–415
Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G (2000)The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest 118:1690–1695
Weisinger JR, Bellorín-Font E (1998) Magnesium and phosphorus. Lancet 352:391–396
Rubeiz GJ, Thill-Baharozian M, Hardie D, Carlson RW (1993) Association of hypomagnesemia and mortality in acutely ill medical patients. Crit Care Med 21:203–209
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. New Engl J Med 345:1359–1367
Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P (2003) Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 31:359–366
Finney SJ, Zekveld C, Elia A, Evans TW (2003) Glucose control and mortality in critically ill patients. JAMA 290:2041–2047
Connolly J, Boyd R, Calvin J (1962) The protective effect of hypothermia in cerebral ischemia: experimental and clinical application by selective brain cooling in the human. Surgery 52:15–24
Schwartz AE, Stone JG, Finck AD, Sandhu AA, Mongero LB, Adams DC, Jonassen AE, Young WL, Michler RE (1996) Isolated cerebral hypothermia by single carotid artery perfusion of extracorporeally cooled blood in baboons. Neurosurgery 39:577–581
Gelman B, Schleien CL, Lohe A, Kuluz JW (1996) Selective brain cooling in infant piglets after cardiac arrest and resuscitation. Crit Care Med 24:1009–1017
Natale JA, D’Alecy LG (1989) Protection from cerebral ischemia by brain cooling without reduced lactate accumulation in dogs. Stroke 20:770–777
Xiao F, Safar P, Alexander H (1995) Peritoneal cooling for mild cerebral hypothermia after cardiac arrest in dogs. Resuscitation 30:51–59
O’Donnel J, Axelrod P, Fisher C, Lorber B (1997) Use and effectiveness of hypothermia blankets for febrile patients in the intensive care unit. Clin Infect Dis 24:1208–1213
Georgiadis D, Schwarz S, Kollmar R, Schwab S (2001) Endovascular cooling for moderate hypothermia in patients with acute stroke. Stroke 32:2550–2553
Doufas AG, Akca O, Barry A, petrusca DA, Suleman MI, Morioka N, Guarnaschelli JJ, Sessler DI (2002) Initial experience with a novel heat-exchanging catheter in neurosurgical patients. Anesth Analg 95:1752–1756
Schmutzhard E, Engelhardt K, Beer R, Brössner G, Pfausler B, Spiss H, Unterberger I, Kampfl A (2002) Safety and efficacy of a novel intravascular cooling device to control body temperature in neurologic intensive care patients: a prospective pilot study. Crit Care Med 30:2481–2488
Andrews PJD, Harris BA (2002) The rationale for selective brain cooling. Year book of intensive care and emergency medicine. Vincent JL (ed) Springer Verlag, Berlin Heidelbrg New York, pp 738–747
Mayer SA, Commichau C, Scarmeas N, Presciutti M, Bates J, Copeland D (2000) Clinical trial of an air-circulating cooling blanket for fever control in critically ill neurologic patients. Neurology 56:292–298
Kammersgaard LP, Rasmussen BH, Jørgensen HS, Reith J, Weber U, Olsen TS (2000) Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: a case-control study: the Copenhagen Stroke Study. Stroke 31:2251–2256
Bernard S, Buist M, Monteiro O, Smith K (2003) Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 56:9–13
Tarnow-Mordi WO, Hau C, Warden A, Shearer AJ (2000) Hospital mortality in relation to staff workload: a 4-year study in an adult intensive-care unit. Lancet 356:185–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polderman, K.H. Application of therapeutic hypothermia in the intensive care unit. Intensive Care Med 30, 757–769 (2004). https://doi.org/10.1007/s00134-003-2151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2151-y