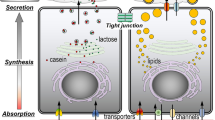
Abstract
The ATP-binding cassette (ABC) efflux transporter ABCG2 represents the main route for active secretion of drugs and toxins across the blood–milk barrier, thereby producing a potential health risk for dairy consumers through formation of relevant residues in milk. However, no suitable in vitro model is as yet available to systematically investigate ABCG2-mediated transport of xenobiotics into milk of dairy animals. We recently cloned ABCG2 from the lactating mammary gland of dairy cows (bABCG2) and goats (cABCG2). Thus, the objective of this study was to generate a suitable blood–milk barrier in vitro model using polarized MDCKII monolayers stably expressing mammary bABCG2 or cABCG2. ABCG2 protein was localized by confocal microscopy to the apical and lateral plasma membrane of polarized MDCKII cells. Intact barrier function of MDCKII-bABCG2 and MDCKII-cABCG2 monolayers was confirmed by determination of cell permeability of transcellular marker propranolol and paracellular marker atenolol which was ≤1 %. In flux assays, ABCG2 substrate 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) showed preferential basolateral to apical (B > A) transport in ABCG2-MDCKII cells. This apically directed PhIP transport was significantly inhibited by ABCG2 inhibitor fumitremorgin C (FTC) or the flavonoid equol. PhIP B > A transport in MDCKII-bABCG2 monolayers was additionally decreased by ABCG2 inhibitor Ko143. The fluoroquinolone antibiotic enrofloxacin was identified as a substrate of ruminant mammary ABCG2. The analgesic drug sodium salicylate was shown to be substrate of bABCG2 but not of cABCG2. Thus, the generated mammary ABCG2-expressing MDCKII cells represent a valuable tool to study active secretion of drugs and toxins into milk.






Similar content being viewed by others
Abbreviations
- ABCG2:
-
ATP-binding cassette subfamily G member 2
- DAPI:
-
4′-6-Diamidino-2-phenylindole
- ER:
-
Efflux ratio
- FTC:
-
Fumitremorgin C
- Ko143:
-
3-(3S,6S,12aS)-6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12 a-octahydro-pyrazino [1,2:1,6] pyrido[3,4-b] indol-3-l)-propionic acid tert-butyl ester
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- MEM:
-
Minimum essential medium
- MDCKII:
-
Madin-Darby canine kidney epithelial cells
- P app :
-
Apparent permeability coefficient
- PhIP:
-
2-Amino-1-methyl-6-phenylimidazo [4,5-b]pyridine
- SDS:
-
Sodiumdodecylsulfate
- TEER:
-
Transepithelial electrical resistance
- ZO-1:
-
Tight-junction-associated zona occludens 1 protein
References
Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1:417–425
Alvarez AI, Real R, Pérez M, Mendoza G, Prieto JG, Merino G (2010) Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci 99:598–617
Braun A, Hämmerle S, Suda K, Rothen-Rutishauser B, Günthert M, Krämer SD, Wunderli-Allenspach H (2000) Cell cultures as tools in biopharmacy. Eur J Pharm Sci 11:S51–S60
Cho MJ, Thompson DP, Cramer CT, Vidmar TJ, Scieszka JF (1989) The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier. Pharm Res 6:71–77
Deli MA (2007) Blood-brain barrier models. In: Lajtha A, Reith MEA (eds) Handbook of neurochemistry and molecular neurobiology, neural membrane and transport, 3rd edn. Springer, New York, pp 29–55
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95:15665–15670
Enokizono J, Kusuhara H, Sugiyama Y (2007) Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol 72:967–975
Gallo P, Fabbrocino S, Vinci F, Fiori M, Danese V, Serpe L (2008) Confirmatory identification of sixteen non-steroidal anti-inflammatory drug residues in raw milk by liquid chromatography coupled with ion trap mass spectrometry. Rapid Commun Mass Spectrom 22:841–854
Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, Lindmark T, Mabondzo A, Nilsson JE, Raub TJ, Stanimirovic D, Terasaki T, Oberg JO, Osterberg T (2005) In vitro models for the blood-brain barrier. Toxicol In Vitro 19:299–334
González-Lobato L, Real R, Prieto JG, Alvarez AI, Merino G (2010) Differential inhibition of murine Bcrp1/Abcg2 and human BCRP/ABCG2 by the mycotoxin fumitremorgin C. Eur J Pharmacol 644:41–48
Halwachs S, Schaefer I, Seibel P, Honscha W (2011) Antiepileptic drugs reduce the efficacy of methotrexate chemotherapy through accelerated degradation of the reduced folate carrier by the ubiquitin-proteasome pathway. Chemotherapy 57:345–356
Halwachs S, Wassermann L, Lindner S, Zizzadoro C, Honscha W (2013) The fungicide prochloraz and environmental pollutant dioxin induce the ABCG2 transporter in bovine mammary epithelial cells by the arylhydrocarbon receptor signaling pathway. Toxicol Sci 131:491–501
Hegedus C, Szakács G, Homolya L, Orbán TI, Telbisz A, Jani M, Sarkadi B (2009) Ins and outs of the ABCG2 multidrug transporter: an update on in vitro functional assays. Adv Drug Deliv Rev 61:47–56
Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR (1999) MDCK (Madin-Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci 88:28–33
Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH (2005) The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med 11:127–129
Kaartinen L, Salonen M, Alli L, Pyörälä S (1995) Pharmacokinetics of enrofloxacin after single intravenous, intramuscular and subcutaneous injections in lactating cows. J Vet Pharmacol Ther 18:357–362
Kneuer C, Honscha KU, Honscha W (2005) Rat reduced-folate carrier-1 is localized basolaterally in MDCK kidney epithelial cells and contributes to the secretory transport of methotrexate and fluoresceinated methotrexate. Cell Tissue Res 320:517–524
Krishnamurthy P, Schuetz JD (2006) Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 46:381–410
Larger P, Altamura M, Catalioto RM, Giuliani S, Maggi CA, Valenti C, Triolo A (2002) Simultaneous LC-MS/MS Determination of Reference Pharmaceuticals as a method for the characterization of the Caco-2 cell monolayer absorption properties. Anal Chem 74:5273–5281
Lindner S, Halwachs S, Wassermann L, Honscha W (2013) Expression and subcellular localization of efflux transporter ABCG2/BCRP in important tissue barriers of lactating dairy cows, sheep and goats. J Vet Pharmacol Ther (in press)
Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE (2000) The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J Cell Sci 113:2011–2021
Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, De Keukeleire D (2003) Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 784:137–144
Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG (2006) Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos 34:690–695
Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH (2005) Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther 312:144–152
Polishchuk R, Di Pentima A, Lippincott-Schwartz J (2004) Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6:297–307
Poller B, Wagenaar E, Tang SC, Schinkel AH (2011) Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm 8:571–582
Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI (2006) Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther 29:279–287
Rabindran SK, Ross DD, Doyle LA (2000) Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res 60:47–50
Real R, Egido E, Pérez M, González-Lobato L, Barrera B, Prieto JG, Alvarez AI, Merino G (2011) Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: interaction with ivermectin. J Vet Pharmacol Ther 34:313–321
Rothen-Rutishauser B, Krämer SD, Braun A, Günthert M, Wunderli-Allenspach H (1998) MDCK cell cultures as an epithelial in vitro model: cytoskeleton and tight junctions as indicators for the definition of age-related stages by confocal microscopy. Pharm Res 15:964–971
Subramanian VS, Marchant JS, Said HM (2008) Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol 294:C233–C240
van Herwaarden AE, Schinkel AH (2006) The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci 27:10–16
van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH (2003) The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res 63:6447–6452
Wang Q, Strab R, Kardos P, Ferguson C, Li J, Owen A, Hidalgo IJ (2007) Application and limitation of inhibitors in drug-transporter interaction studies. Int J Pharm 356:12–18
Wassermann L, Halwachs S, Lindner S, Honscha KU, Honscha W (2013) Determination of functional ABCG2 activity and assessment of drug-ABCG2 interactions in dairy animals using a novel MDCKII in vitro model. J Pharm Sci 102:772–784
Xia CQ, Liu N, Yang D, Miwa G, Gan LS (2005) Expression, localization, and functional characteristics of breast cancer resistance protein in Caco-2 cells. Drug Metab Dispos 33:637–643
Zhao X, Lacasse P (2008) Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci 86:57–65
Acknowledgments
We kindly thank Pablo Steinberg (Institute for Food Toxicology and Analytical Chemistry, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany) for donating PhIP.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wassermann, L., Halwachs, S., Baumann, D. et al. Assessment of ABCG2-mediated transport of xenobiotics across the blood–milk barrier of dairy animals using a new MDCKII in vitro model. Arch Toxicol 87, 1671–1682 (2013). https://doi.org/10.1007/s00204-013-1066-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1066-9