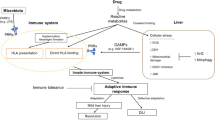
Abstract
There has been a substantial interest in drug-induced liver injury (DILI) recently. National Institutes of Health has sponsored a multicenter study in the USA for the last 10 years, which has collected valuable information in this context. Idiosyncratic DILI is like other adverse effects of drugs underestimated and underreported in most epidemiological studies. A recent prospective population-based study from Iceland found a crude incidence of approximately 19 cases per 100,000 and year. Antibiotic is the class of drugs most commonly implicated in patients with DILI. Amoxicillin–clavulanate continues to be the most commonly implicated agent occurring in approximately 1 out of 2,300 users. Drugs with the highest risk of DILI in the Icelandic study were azathioprine and infliximab. Although rare, statin-induced hepatotoxicity has been well documented. Liver injury associated with the use of herbal medicines and dietary supplements seems to be increasing. Information on the documented hepatotoxicity of drugs has recently been made easier by a website available in the public domain: LiverTox (http://livertox.nlm.nih.gov). Unfortunately, at the current time, pre-therapy risk assessment for DILI in the individual patient is difficult but previous well-documented hepatotoxicity is usually a contraindication for a subsequent treatment with the same drug.
Similar content being viewed by others
References
Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, Kenna JG, Caldwell J, Day CP (2004) Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 39:1430–1440
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H et al (2011) Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 89:806–815
Andrade RJ, Lucena MI, Fernandez MC et al (2005) Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 129:512–521
Benichou C, Danan G, Flahault A (1993) Causality assessment of adverse reactions to drugs-II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 46:1331–1336
Björnsson E, Olsson R (2005) Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 42:481–489
Björnsson E, Davidsdottir L (2009) The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatology 50:511–517
Björnsson E, Kalaitzakis E, Olsson R (2007) The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther 25:1411–1421
Björnsson E, Jacobsen EI, Kalaitzakis E (2012) Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 56:374–380
Björnsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S (2013) Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144:1419–1425
Björnsson ES, Gunnarsson BI, Gröndal G, Jonasson JG, Einarsdottir R, Ludviksson BR, Gudbjörnsson B, Olafsson O (2014) The risk of drug-induced liver injury from Tumor Necrosis Factor (TNF)-alpha-antagonists. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2014.07.062
Chalasani N, Fontana RJ, Bonkovsky HL et al (2008) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135:1924–1934
Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA (2005) Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med 118:618–624
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, Graham J, Park BK, Dillon JF, Bernal W, Cordell HJ, Pirmohamed M, Aithal GP, Day CP (2009) Nat Genet 41:816–819
Danan G, Benichou C (1993) Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46:1323–1330
Davies MH, Harrison RF, Elias E, Hübscher SG (1994) Antibiotic-associated acute vanishing bile duct syndrome: a pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatol 20:112–116
de Abajo FJ, Montero D, Madurga M, de García Rodríguez LA (2004) Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol 58(1):71–80
De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E (2006) Drug induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther 24(8):1187–1195
Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK (2010) Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 105:2396–2404
Fairley CK, McNeil JJ, Desmond P, Smallwood R, Young H, Forbes A, Purcell P, Boyd I (1993) Risk factors for development of flucloxacillin associated jaundice. BMJ 306:233–235
Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM (2005) Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 50:1785–1790
Fontana R, Seef LB, Andrade RJ, Bjornsson E, Day CP, Serrano J, Hoofnagle JH (2010) Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology 52:730–742
Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, Serrano J, Lee WM, Chalasani N, Stolz A, Davern T, Talwakar JA (2014) Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 147(1):96–108
García Rodríguez LA, Stricker BH, Zimmerman HJ (1996) Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med 156:1327–1332
García Rodríguez LA, Ruigómez A, Jick H (1997) A review of epidemiologic research on drug-induced acute liver injury using the general practice research data base in the United Kingdom. Pharmacotherapy 17:721–728
Ghabril M, Bonkovsky HL, Kum C et al (2013) Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol 11:558–564
Hussaini SH, O’Brien CS, Despott EJ, Dalton HR (2007) Antibiotic therapy: a major cause of drug-induced jaundice in southwest England. Eur J Gastroenterol Hepatol 19:15–20
Ibanez L, Perez E, Vidal X, Laporte JR (2002) Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol 37:592–600
Lammert C, Einarsson S, Saha C et al (2008) Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology 47:2003–2009
Lee WM (2008) Acetaminophen-related acute liver failure in the United States. Hepatol Res 38:S3–S8
Lucena MI, Andrade RJ, Fernández MC, Pachkoria K, Pelaez G, Durán JA et al (2006) Determinants of the clinical expression of amoxicillin–clavulanate hepatotoxicity: a prospective series from Spain. Hepatology 44:850–856
Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, Hoofnagle JH, Chalasani N (2014) Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. doi:10.1002/hep.27542
Navarro VJ, Lucena MI (2014) Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis 34(2):172–193
Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R (2014) Liver injury from herbals and dietary supplements in the U.S. drug-induced liver injury network. Hepatology 60(4):1399–1408
Olsson R, Wiholm BE, Sand C, Hultcrantz R, Myrhed M (1992) Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J Hepatol 15:154–161
Pauli-Magnus C, Meier PJ (2006) Hepatobiliary transporters and drug-induced cholestasis. Hepatology 44:778–787
Pérez Gutthann S, García Rodríguez LA (1993) The increased risk of hospitalizations for acute liver injury in a population with exposure to multiple drugs. Epidemiology 4(6):496–501
Reuben A, Koch DG, Lee WM (2010) Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 52:2065–2076
Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P (2004) Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl 10:1018–1023
Russo MW, Scobey M, Bonkovsky HL (2009) Drug-induced liver injury associated with statins. Review. Semin Liver Dis 29:412–422
Sgro C, Clinard F, Ouazir K et al (2002) Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36:451–455
Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, Baik GH, Kim JB, Kweon YO, Kim BI, Kim SH, Kim IH, Kim JH, Nam SW, Paik YH, Suh JI, Sohn JH, Ahn BM, Um SH, Lee HJ, Cho M, Jang MK, Choi SK, Hwang SG, Sung HT, Choi JY, Han KH (2012) A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol 107(9):1380–1387
Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, Hunt CM, Freston JW (2010) Unified list of drugs causing hepatotoxicity and the reporting frequency of liver events in the WHO vigibaset: international collaborative work. Drug Saf 33:503–522
Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, Lim SG (2007) Drug-induced liver injury at an Asian center: a prospective study. Liver Int 27:465–474
Turner IB, Eckstein RP, Riley JW, Lunzer MR (1989) Prolonged hepatic cholestasis after flucloxacillin therapy. Med J Aust 151:701–705
Wei G, Bergquist A, Broome U, Lindgren S, Wallerstedt S, Almer S, Sangfeldt P, Danielsson A Sandberg-Gertzen H, Loof L, Prytz H, Björnsson E (2007) Acute liver failure in Sweden: etiology and prognosis. J Internal Med 262:393–401
Zimmerman HJ (1999) Drug-induced liver disease. In: Sciff ER, Sorrell MF, Maddrey WC (eds) Schiff´s diseases of the liver, 8th edn. Lippincott-Raven Publishers, Philadelphia, pp 973–1064
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Björnsson, E.S. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 89, 327–334 (2015). https://doi.org/10.1007/s00204-015-1456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1456-2