Abstract
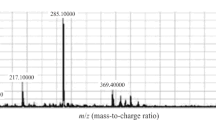
Letrozole is an efficient endocrine treatment of postmenopausal breast cancer, however, not all patients benefit from this treatment, and moreover, severe side-effects like arthralgia frequently lead to discontinuation. To better understand inter-individual variability in drug response and side-effects, plasma analysis of steady-state concentrations of letrozole and its major metabolites is crucial. We developed a rapid, sensitive, and specific method for the simultaneous quantification of letrozole and its metabolites 4,4′-(hydroxymethylene)dibenzonitrile (carbinol) and bis(4-cyanophenyl)methyl hexopyranosiduronic acid (carbinol-gluc) by UHPLC-ESI-MS/MS using in-house synthesized, stable isotope-labeled internal standards. Following solid-phase extraction in BondElut C18 96-well plates, the analytes were separated on a ZORBAX Eclipse XDB-C18 column (1.8 μm, 4.6 × 50 mm) with a gradient of acetonitrile in 0.1% acetic acid in water and detected on a triple quadrupole mass spectrometer with electrospray ionization in the multiple reaction monitoring mode. Lower limits of quantification were 20, 0.2, and 2 nM for letrozole, carbinol, and carbinol-gluc, respectively. The assay has been validated according to FDA guidance and applied to the analysis of 20 plasma samples of postmenopausal breast cancer patients treated with 2.5 mg of letrozole per day. Mean plasma levels (±SD) were 366 ± 173, 0.38 ± 0.09, and 34 ± 12 nM for letrozole, carbinol, and carbinol-gluc, respectively. Our rapid and sensitive mass spectrometry based method enables future pharmacokinetic investigations of letrozole outcome.

LC-MS/MS analysis of letrozole and its metabolites in human plasma



Similar content being viewed by others
References
Mouridsen HT (2006) Curr Med Res Opin 22:1609–1621
Din OS, Dodwell D, Wakefield RJ, Coleman RE (2010) Breast Cancer Res Treat 120:525–538
Lazarus P, Sun D (2010) Drug Metabol Rev 42:182–194
Murai K, Yamazaki H, Nakagawa K, Kawai R, Kamataki T (2009) Xenobiotica 39:795–802
Sioufi A, Gauducheau N, Pineau V, Marfil F, Jaouen A, Cardot JM, Godbillon J, Czendlik C, Howald H, Pfister C, Vreeland F (1997) Biopharm Drug Dispos 18:779–789
Haberl M, Anwald B, Klein K, Weil R, Fuss C, Gepdiremen A, Zanger UM, Meyer UA, Wojnowski L (2005) Pharmacogenet Genom 15:609–624
Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W (2011) Pharmacogenomics J 11:274–286
Zanger UM, Turpeinen M, Klein K, Schwab M (2008) Anal Bioanal Chem 392:1093–1108
Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA (2011) Clin Pharmacol Ther 90:693–700
Pfister CU, Duval M, Godbillon J, Gosset G, Gygax D, Marfil F, Sioufi A, Winkler B (1994) J Pharm Sci 83:520–524
Marfil F, Pineau V, Sioufi A, Godbillon SJ (1996) J Chromatogr B Biomed Appl 683:251–258
Beer B, Schubert B, Oberguggenberger A, Meraner V, Hubalek M, Oberacher H (2010) Anal Bioanal Chem 398:1791–1800
Mazzarino M, Botre F (2006) Rapid Commun Mass Spectrom 20:3465–3476
Kolmonen M, Leinonen A, Pelander A, Ojanpera I (2007) Anal Chim Acta 585:94–102
Mareck U, Sigmund G, Opfermann G, Geyer H, Thevis M, Schanzer W (2005) Rapid Commun Mass Spectrom 19:3689–3693
Guidance for Industry—bioanalytical method validation (2001) U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD, USA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed on 15 Sept 2011
Leigh WJ, Arnold DR, Humphreys RWR, Wong PC (1980) Can J Chem 58:2537–2549
Lang M (Ciba-Geigy Corporation, Ardsley, NY) Tetrazolyl substituted benzonitriles and anti-tumor use thereof. U.S. Patent 5,073,574, December 17, 1991
Palle RA, Kalaria AJ, Shelke SA (Dr. Reddy’s Laboratories, Inc., Bridgewater, NJ) Process for preparing letrozole. U.S. Patent Application 20070100149, May 3, 2007
Barzaghi M, Gamba A, Oliva C (1988) J Chem Soc Faraday Trans 1(84):3279–3291
Conrow RB, Bernstein S (1971) J Org Chem 36:863–870
Acknowledgments
We thank the German Tamoxifen and AI clinicians group for recruiting letrozole-treated patients. This work was supported by grants from the Hans L. Merkle Foundation, the Robert Bosch Foundation (Stuttgart, Germany), and from the Federal Ministry for Education and Research (BMBF, Berlin, Germany; 03IS2061C and 01ZP0502).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 246 kb)
Rights and permissions
About this article
Cite this article
Precht, J.C., Ganchev, B., Heinkele, G. et al. Simultaneous quantitative analysis of letrozole, its carbinol metabolite, and carbinol glucuronide in human plasma by LC-MS/MS. Anal Bioanal Chem 403, 301–308 (2012). https://doi.org/10.1007/s00216-012-5813-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5813-1