Abstract
Objectives
To determine whether ingestion of milk thistle affects the pharmacokinetics of indinavir.
Methods
We conducted a three-period, randomized controlled trial with 16 healthy participants. We randomized participants to milk thistle or control. All participants received initial dosing of indinavir, and baseline indinavir levels were obtained (AUC0-8) (phase I). The active group were then given 450 mg milk-thistle extract capsules to be taken t.i.d. from day 2 to day 30. The control group received no plant extract. On day 29 and day 30, indinavir dosing and sampling was repeated in both groups as before (phase II). After a wash-out period of 7 days, indinavir dosing and sampling were repeated as before (phase III).
Results
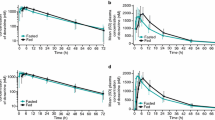
All participants completed the trial, but two were excluded from analysis due to protocol violation. There were no significant between-group differences. Active group mean AUC0-8 indinavir decreased by 4.4% (90% CI, −27.5% to −26%, P=0.78) from phase I to phase II in the active group, and by 17.3% (90% CI, −37.3% to +9%, P=0.25) in phase III. Control group mean AUC0-8 decreased by 21.5% (90% CI, −43% to +8%, P=0.2) from phase I to phase II and by 38.5% (90% CI, −55.3% to −15.3%, P=0.01) of baseline at phase III. To place our findings in context, milk thistle–indinavir trials were identified through systematic searches of the literature. A meta-analysis of three milk thistle–indinavir trials revealed a non-significant pooled mean difference of 1% in AUC0-8 (95% CI, −53% to 55%, P=0.97).
Conclusions
Indinavir levels were not reduced significantly in the presence of milk thistle.



Similar content being viewed by others
References
Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J (2000) Indinavir concentrations and St John’s wort. Lancet 355:547–548
de Maat MM, Hoetelmans RM, Matht RA et al. (2001) Drug interaction between St John’s wort and nevirapine. Aids 15:420–421
The 8 most important supplements for people on HAART (1999) STEP perspect 99:18
Venkataramanan R, Ramachandran V, Komoroski BJ, Zhang S, Schiff PL, Strom SC (2000) Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab Dispos 28:1270–1273
Foster BC, Drouin CE, Livesey J, Arenson JT, Mills E (2004) In vitro activity of milk thistle against cytochrome P450 isozymes. Int JNM 1:49–52
Piscitelli SC, Formentini E, Burstein AH, Alfaro R, Jagannatha S, Falloon J (2002) Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy 22:551–556
DiCenzo R, Shelton M, Jordan K et al (2003) Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy 23:866–870
Mills EJ, Perri D, Phillips E, Koren G (2004) Natural health products-HIV drug interactions: a systematic review. Int J STD AIDS (in press)
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J (2002) The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis 34:234–238
Gallicano K, Foster B, Choudhri S (2003) Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol 55:199–202
Mills EJ, Montori VM, Wu P, Gallicano KD, Clarke M, Guyatt G (2004) Herb–drug interactions: a systematic review of clinical trials. Archf Intern Med [Abstract: presented at the 10th Annual Symposium on Complementary Health Care. London, UK, November 2003]
Sackett DL (2001) Why randomized controlled trials fail but needn‘t: 2. Failure to employ physiological statistics, or the only formula a clinician-trialist is ever likely to need (or understand!). CMAJ 165:1226–1237
van Heeswijk RP, Veldkamp A, Mulder JW et al (2001) Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir Ther 6:201–229
Choo EF, Leake B, Wandel C et al (2000) Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos 28:655–660
Jones K, Bray PG, Khoo SH et al (2001) P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance. Aids 15:1353–1358
Huisman MT, Smit JW, Crommentuyn KM et al.(2002) Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. Aids 16:2295–2301
Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023
Khaliq Y (1998) Effects og nelfinavir on short and long term plasma exposure of saquinavir in hard gel capsule during bid and tid dosing intervals. In: 4th international congress on drug therapy and HIV Infection. Abstract 327
Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R et al (2000) Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol Toxicol 86:250–256
Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS (1998) Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med 158:2257–2264
Liu JP, Manheimer E, Yang M (2004) Herbal medicines for treating HIV infection and AIDS: a Cochrane systematic review. In: Paper presented at: XV international AIDS Conference, Bangkok, Thailand
Acknowledgements
This study was supported by The Ontario HIV Treatment Network. Edward Mills conceptualized the study, obtained funding, conducted the trial and wrote the manuscript. Kumanan Wilson co- conceptualized the study, obtained funding, conducted the trial and wrote the manuscript. Mike Clarke assisted in trial design, provided critical insights in conduct and co-wrote the manuscript. Brian Foster assisted in obtaining funding, analysis of the plant extract and co-wrote the manuscript. Scott Walker assisted in obtaining funding, analysis of blood and co-wrote the manuscript. Beth Rachlis enrolled participants and coordinated the study. Nick DeGroot assisted in trial planning, coordination and co-wrote the manuscript. Victor Montori conducted the meta-analysis. Wayne Gold assisted in trial monitoring and conducted all patient-related management. Elizabeth Phillips assisted in obtaining funding, design of trial, interpretation of results and co-wrote the manuscript. Stephen Myers assisted in patient management and trial planning. He co-wrote the manuscript. Keith Gallicano assisted in obtaining funding, trial design, analysis of blood and statistics. He co-wrote the manuscript. The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mills, E., Wilson, K., Clarke, M. et al. Milk thistle and indinavir: a randomized controlled pharmacokinetics study and meta-analysis. Eur J Clin Pharmacol 61, 1–7 (2005). https://doi.org/10.1007/s00228-004-0843-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0843-z