Abstract
Objective
Measurement of plasma methadone concentration to investigate the rate of clearance of methadone prescribed for heroin dependence in the first, second and third trimesters of pregnancy. A secondary objective was to evaluate the outcome of pregnancy.
Methods
Longitudinal within subject study of nine pregnant opioid dependent subjects prescribed methadone at the Leeds Addiction Unit, an outpatient community based treatment centre. Plasma concentration versus time data for methadone was collected during each trimester and post-partum for our subjects. Data was available for the first and second trimesters for 4/9 cases. All but one of the subjects provided data during the third trimester and data post-partum was collected from three respondents. Measurements of methadone levels in plasma were carried out using high performance liquid chromatography (HPLC).
Results
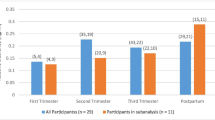
Trough mean plasma methadone concentrations reduced as the pregnancies progressed from 0.12 mg/L (first trimester) to 0.07 mg/L (third trimester). The weight-adjusted clearance rates gradually increased from a mean of 0.17 to 0.21 L/hr/kg during pregnancy, although patterns differed substantially between the nine women. An assessment of relative clearance of methadone using two patients for whom we have had all three CL values (trimester 1–3) demonstrated notable change of CL (P=0.056) over time. Eight of our subjects delivered (3 males), within two weeks of their due date the ninth (male) was premature (21 days). The mean length of gestation was 39.7 weeks (SD=10 days) and none of the neonates met criterion for ‘low birth weight’ mean 3094, SD 368 g). Five neonates spent time (0.5–28 days) in a special care baby unit (SCUBU) and 4 of these displayed signs of methadone withdrawal.
Conclusions
General Practitioners and hospital doctors should recognise the significant benefits of prescribing methadone for heroin-dependent women during pregnancy. We recommend that if a pregnant opioid user complains of methadone withdrawal symptoms (i.e. that the methadone dose does not “hold” them) the prescribing clinician takes this observation seriously and considers a more detailed assessment. Further work on key factors undergoing changes during pregnancy accounting for differences in methadone metabolism in the mother, fetus and neonate are required.

Similar content being viewed by others
References
Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H (2001) Psychiatric Morbidity among adults living in private households, 2000. Office for National Statistics. The Stationary Office, London
Ellwood DA, Sutherland P, Kent C, O'Connor M (1987) Maternal narcotic addiction: pregnancy outcome in patients managed by a specialized drug-dependency antenatal clinic. Aust & NZ Obsett and Gynae 27:92–98
Finnegan LP (1983) Clinical perinatal and development effects of methadone. In: Cooper JR, Altman F, Brown BS, Czechowicz D (eds) Research on the Treatment of Narcotic Addiction: State of the Art. Maryland: National Institute on Drug Abuse, USA
Finnegan L P (1991) Treatment issues for opioid-dependent women during the perinatal period. J Psycho Drugs 23:191–201
Salerno LJ (1979) Prenatal care. In: Rementeria J (ed) Drug Abuse in Pregnancy and Neonatal Effects St Louis, CV, USA: Mosby Company pp.19–29
Giles W, Patterson T, Sanders F, Batey R, Thomas D, Collins J (1989) Outpatient methadone programme for pregnant heroin using women. Aust & NZ Obsett and Gynae 29:225–229
Householder J, Hatcher R, Burns W, Chasnoff I (1982) Infants born to narcotic-addicted mothers. Psych Bull 92:453–468
Finnegan LP (1980) (ed) Drug dependence in pregnancy: clinical management of mother and child. London: Castle House 1980
Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, Langer M, Aschaver HN (2000) Treatment of opioid-dependent pregnant women with buprenorphine. Addiction 95:239–244
Suffet F, Brotman RA (1984) Comprehensive care program for pregnant addicts: obstetrical, neonatal, and child development outcomes. Inter J of the Addict 19:199–219
Wilson GS (1989) Clinical studies of infants and children exposed prenatally to heroin. Ann NY Acad Sci 562:183–194
Pond SM, Kreek MJ, Tong TG, Raghunath J, Benowitz NL (1985) Altered methadone pharmacokinetics in methadone-maintained pregnant women. J Pharmacol Exper Therap 233:1–6
Mackie-Ramos RL, Rice JM (1988) Group psychotherapy with methadone-maintained pregnant women. J Subst Abuse Treat 5:151–161
Edelin KC, Gurganious L, Golar K, Oellerich D, Kyei-Aboagye K, Hamid M (1988) Methadone maintenance in pregnancy: consequences to care and outcome. Obsett Gynae 71:399–404
Offidani C, Chiarotti M, De Giovanni N, Falasconi AM (1986) Methadone in pregnancy: clinical-toxicological aspects. J Tox Clin Toxicol 24:295–303
Jarvis MA, Wu-Pong S, Kniseley JS, Schnoll SH (1999) Alterations in methadone metabolism during late pregnancy. J Addict Dis 18:51–61
Department of Health (1999) Drug Misuse and Dependence-Guidelines on Clinical Management. The Stationery Office, London, UK
Wolff K, Rostami-Hodjegan A, Hay AW, Raistrick D, Tucker G (2000) Population-based pharmacokinetic approach for methadone monitoring of opiate addicts: potential clinical utility. Addiction 95:1771–1783
Eap CB, Buclin T, Baumann P (2002) Interindividual variability of the clinical pharmacokinetics of methadone, Implications for the treatment of opioid dependence. Clin Pharmacol 41:1153–1193
Kharasch ED, Hoffer C, Whittington D, Sheffels P (2004) Role of heptatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Cli Pharmacol & Therap 76:250–269
Lewis DFV, Ioannides C, Parke DV, Schulte-Hermann R (2000) Quantitative structure activity relationships in a series of endogenous and synthetic steroids exhibiting induction of CYP3A activity and hepatomegaly associated with increased DNA synthesis. J Steroid Biochem Mol Biol 74:179–185
Tracy TS, Venkataramanan R, Glover DD, Caritis SN (2005) Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Amer J Obstet Gynae 192:633–639
Wadelius M, Darj E, Frenne G, Rane A (1997) Induction of CYP2D6 in pregnancy. J Clin Pharmacol Therap 62:400–407
Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL (2005) Bidirectional transfer of methadone across human placenta. Biochem Pharmacol 69:187–197
Clarke K, Formby J (2000) Feeling good, doing fine. Druglink 15:10–13
Blinick G, Jerez E, Walach RC (1973) Methadone Maintenance, Pregnancy and progeny. JAMA 225:477–479
Wolff K, Rostami-Hodjegan A, Shires S, Hay A W, Feely M, Calvert R, Raistrick D, Tucker G T (1997) The pharmacokinetics of methadone in healthy subjects and opiate users. Brit J Clin Pharm 44:325–334
Rostami-Hodjegan A, Wolff K, Hay AW, Raistrick D, Calvert R, Tucker GT (1999) Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Brit J Clin Pharmacol 48:43—52
Swift RM, Dudley M, DePetrillo P, Camara P, Griffiths W (1989) Altered methadone pharmacokinetics in pregnancy: implications for dosing. J Subst Abuse 1:453–460
Torimoto N, Ishii I, Hata M, Nakamura H, Imada H, Ariyoshi N, Ohmori S, Igarashi T, Kitada M (2003) Direct interaction between substrates and endogenous steroids in the active site may change activity of Cytochrome P450 3A4. Biochem 42:15068–15077
Sarkar M, Vadlamuri V, Ghosh S, Glover DD (2003) Expression and cyclic variability of CYP3A4 and CYP3A7 isoforms in human endometrium and cervix during the menstrual cycle. Drug Metabolism & Disposition 31(1):1–6
Williams ET, Leyk M, Wrighton SA, Davies PJ, Loose DS, Shipley GL, Strobel HW (2004) Estrogen regulation of the Cytochrome P450 3A subfamily in humans. J Pharmacol Exper Therap 311:728–735
Alcorn J, McNamara PJ (2002) Ontogeny of hepatic and renal systematic clearance pathways in infants. Clin Pharmacol 41:959–998
Nanovskaya TN, Deshmukh SV, Nekhayeva IA, Zharikova OL Hankins GD, Ahmed MS (2004) Methadone metabolism in the human placenta. Biochem Pharmacol 68:583–591
Matsunaga T, Maruyama M, Harada E, Katsuyama Y, Sugihara N, Ise H, Negishi N, Ikeda U, Ohmori S (2004) Expression and induction of CYP3As in human fetal hepatocytes. Biochem Biophy Res Comm 318(2):428–434
Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, Hamman MA, Hall SD, Wrighton SA (2002) Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Disposition 30:883–891
Ring JA, Ghabrial H, Ching MS, Smallwood RA, Morgan DJ (1999) Fetal hepatic drug elimination. J Pharmacol Therap 84:429–445
Finnegan LP, Kaltenbach K (1992) Neonatal abstinence syndrome, In: Hoekeman RA, Friedman SB, Nelson N, Seidel HM (eds) Primary Pediatric Care 2nd edn, 1367–1378, St Louis, CV, Mosby, USA
Levy M, Spino M (1993) Neonatal withdrawal syndrome: associated drugs and pharmacologic management, Pharmacotherapy 13:202–211
Kandall SR (1995) Treatment options for drug-exposed infants, NIDA Research Monograph 149:58–77
Romach MK, Piafsky KM, Abel JG, Khouw V, Sellers EM (1981) Methadone binding to orosomucoid (alpha 1-acid glycoprotein): determinat of free fraction in plasma. Clin Pharmacol Ther 29:211–217
Keller F, Griesshammer M, Haussler U, Paulus W, Schwarz A (2001) Pregnancy and renal failure: the case for application of dosage guidelines. Drugs 61:1901–1920
Gerber JG, Rosenkranz S, Segal Y, Aberg J, D'Amico R, Mildvan D, Gulick R, Hughes V, Flexner C, Aweeka F, Hsu A, Gal J, ACTG 401 Study Team (2001) Effect of ritonavir/saquinavir on stereoselective pharmacokinetics of methadone: results of AIDS Clinical Trials Group (ACTG) 401. J Immune Def Syndromes: JAIDS 27:106–153
Foster DJ, Somogyi AA, White JM, Bochner F (2004) Population pharmacokinetics of R-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol 57:742–755
Boulton DW, Arnaud P, Devane CL (2001) Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin Pharmacol Ther 70:48–57
Rodrigo M, Ortega I, Soengas I, Lean N, Saurez E, Calvo R, Lukas JC (2004) Alpha-1-acid glycoprotein directly effects the pharmacokinetics and the analgesic effect of methadone in the rat beyond protein binding. J of Pharm Sci 93:2836–2850
Department of Health (1993) Challenging Childbirth: Good Practice in Maternity Care. London, Department of Health, The Stationery Office, London, UK
Standing Conference on Drug Abuse (SCODA), Local Government Drugs Forum (1997) Drug using parents: policy guidelines for inter-agency working. London: LGDF
Mounteney J (1999) Drugs, pregnancy & childcare: a guide for professionals. London: ISDD, UK
Nanovskaya TN, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS (2005) Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol 69:583–591
Strolin BM, Baltes EL (2003) Drug metabolism and disposition in children. Fund Clinl Pharmacol 17:281–299
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolff, K., Boys, A., Rostami-Hodjegan, A. et al. Changes to methadone clearance during pregnancy. Eur J Clin Pharmacol 61, 763–768 (2005). https://doi.org/10.1007/s00228-005-0035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0035-5