Abstract
Objective
The 5HT1A receptor agonist sarizotan is in clinical development for the treatment of dyskinesia, a potentially disabling complication in Parkinson’s disease. We investigated the effect of sarizotan on the clinical pharmacokinetics of probe drugs for cytochrome P450 (CYP) to evaluate the risk of CYP-related drug-drug interactions.
Methods
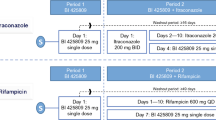
This was a double-blind, randomised, two-period cross-over interaction study with repeated administration of 5 mg sarizotan HCl or placebo b.i.d. for 8 days in 18 healthy volunteers. On day 4, a single dose of 100 mg metoprolol (CYP2D6 probe) was administered. On day 8, single doses of 100 mg caffeine (CYP1A2 probe), 50 mg diclofenac (CYP2C9 probe), 100 mg mephenytoin (CYP2C19 probe) and 7.5 mg midazolam (CYP3A4 probe) were simultaneously applied. Pharmacokinetic parameters for probe drugs and their metabolites in plasma and urinary recovery were determined.
Results
Concentration-time profiles and pharmacokinetic parameters of all probes and their metabolites remained unchanged after co-administration of sarizotan, compared with placebo. Analysis of variance of the area under the plasma concentration-time curve for probe drugs/metabolites, metabolic ratios and urinary excretion resulted in 90% confidence intervals within the acceptance range (0.8–1.25), indicating the absence of drug-drug interactions.
Conclusions
At a dose higher than that intended for clinical use (1 mg b.i.d.), sarizotan had no effect on the metabolism and pharmacokinetics of specific probe drugs for CYP isoenzymes 1A2, 2C19, 2C9, 2D6 and 3A4. Pharmacokinetic interactions with co-administered drugs metabolised by these CYP isoforms are not expected, and dose adjustment of co-administered CYP substrates is not necessary.

Similar content being viewed by others
References
Backman JT, Aranko K, Himberg JJ, and Olkkola KT (1994) A pharmacokinetic interaction between roxithromycin and midazolam. Eur J Clin Pharmacol 46:551–555
Bertilsson Leif, Dahl Marja-Liisa, Dalén Per, Al-Shurbaji Ayman (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53:111–122
Bibbiani F, Oh JD, Chase TN (2001) Sarizotan 5-HT1A agonist improves motor complications in rodent and primate Parkinsonian models. Neurology 57:1829–1934
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King P, Miwa G, Ni L, Kumar G, McLeod J, Obach SR, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton A (2003) The conduct of in vitro and in vivo drug drug interaction studies: A PhRMA perspective. J Clin Pharmacol 43:443–469
Bruce MA, Hall SD, Haehner-Daniels BD, Gorski JC (2001) In vivo effect of clarithromycin on multiple cytochrome P450s. Drug Metab Dispos 29:1023–1028
Ceravolo R, Nuti A, Piccinni A, Dell'Agnello G, Bellini G, Gambaccini G, Dell’Osso L, Murri L, Bonuccelli U (2000) Paroxetine in Parkinson’s disease: effects on motor and depressive symptoms. Neurology 55:1216–1218
EUFEPS Conference report (2001) Optimising drug development: strategies to assess drug metabolism/transporters interaction potential - towards a consensus. Eur J Pharm Sci 13:417–428
Frye F, Matzke GR, Adenoyin A (1997) Validation of the five-drug “Pittsburgh cocktail” approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther 62:365–376
Hewitt NJ, Bühring KU, Dasenbrock J, Haunschild J, Ladstetter B, Utesch D (2001) Studies comparing in vivo:in vitro metabolism of three pharmaceutical compounds in rat, dog, monkey, and human using cryopreserved hepatocytes, microsomes, and collagen gel immobilized hepatocyte cultures. Drug Metab Disp 29:1042–1050
Hoefer C, Galleman D, Fluck F, Buhring KU, Ladestter B, Dolgos H. An in vitro assessment of sarizotan inhibitory and induction potential towards major hepatic cytochromes P450, in preparation
Kay OM, Nicholls B (2000) Clinical Pharmacokinetics of Ropinirole. Clin Pharmacokinet 39:243–254
Kirchheiner J, Meineke I, Müller G, Roots I, Brockmöller J (2002) Contributions of CYP2D6, CYP 2C9 and CYP2C19 to the biotransformation of E-and Z doxepine in healthy volunteers. Pharmacogenetics 12:571–579
Leucuta A, Vlase L, Farcau D, Nanulescu M (2004) No effect of short term ranitidine intake on diclofenac pharmacokinetics. Rom J Gastroenterol 13:306–308
Nelson MV, Berchou RC, Kareti D, LeWitt PA (1990) Pharmacokinetic evaluation of erythromycin and caffeine administered with bromocriptine. Clin Pharmacol Ther 47:694–697
Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H (2004) Multicenter, open-lable, trial of Sarizotan in Parkinson Disease patients with levodopa-induced dyskinesia (the SPLENDID Study) Clin Neuropharmacol 27:58–62
Olanow CW, Watts RL, Koller WC (2001) An algorithm (decision tree) for management of Parkinson’s disease. Treatment guidelines. Neurology 56 (S5):1–88
Palmer JL, Scott RJ, Gibson A, Dickins M, Pleasance S (2001) An interaction between the cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A4). Br J Clin Pharmacol 52:555–561
Regardh CG, Landahl S, Larsson M, Lundborg P, Stehen B, Hoffmann KJ, Langerström PO (1983) Pharmacokinetics of metoprolol and its metabolite α-hydroxymetoprolol in health, non-smoking, elderly individuals. Eur J Clin Pharmacol 24:221–226
Richard IH (2000) Depression in Parkinson's disease. Curr Treat Op Neurol 2:263–274
Scott RJ, Palmer J, Lewis IAS, Pleasance S (1999) Determination of a “GW cocktail” of cytochrome P450 probe substrates and their metabolites in plasma and urine using automated solid phase extraction and fast gradient liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom 13:2305–2319
Suchowersky O (2002) Parkinson's disease: medical treatment of moderate to advanced disease. Curr Neurol Neurosci Rep 2:310–316
Sugiyama Y, Kato Y, Ito K (2002) Quantitative prediction: metabolism, Transport in the liver. In Li A, Sugiyama Y (Eds) Preclinical and clinical evaluation of drug - drug interactions, I S E Press, 108–115
Tamminga WJ, Wemer J, Oosterhuis B, Brakenhoff JP, Gerrits MG, de Zeeuw RA, de Leij LF, Jonkman JH (2001) An optimized methodology for combined phenotyping and genotyping on CYP2D6 and CYP2C19. Eur J Clin Pharmacol 57:143–146
The European Agency for the Evaluation of Medicinal Products, Committee for proprietary medicinal products (1997) Not for Guidance on the investigation of drug interactions (CPMP/EWP/560/95)
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER)(1998) Guidance for Industry: In vivo Drug metabolism/Drug Interaction Studies - Study Design, Data Analysis, and Recommendations for Dosing and Labeling
Wrighton SA, Schuetz EG, Thummel KE, Shen DD, Korzenka KR, Watkins PB (2000) The human CYP3A subfamily practical considerations. Drug Metab Rev 32:339–361
Zhou H, Tong Z, Mc Leod J (2004) “Cocktail “ Approaches and Strategies in Drug Development: Valuable tool or flawed science? J Clin Pharmacol 44:120–134
Zhu B, Ou-Yang DS, Chen XP, Huang SL, Tan ZR, He N, Zhou HH (2001) Assessment of cytochrome P450 activity by a five-drug cocktail approach. Clin Pharmacol Ther 70:455–461, DOI:10.1067/mcp.2001.119813
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krösser, S., Neugebauer, R., Dolgos, H. et al. Investigation of sarizotan’s impact on the pharmacokinetics of probe drugs for major cytochrome P450 isoenzymes: a combined cocktail trial. Eur J Clin Pharmacol 62, 277–284 (2006). https://doi.org/10.1007/s00228-006-0101-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0101-7