Abstract
Objective: A series of studies was undertaken to determine the cytochrome P450 isoform(s) involved in naproxen demethylation and whether this included the same isoforms reported to be involved in the metabolism of other NSAIDs.
Methods: (S)-Naproxen was incubated with human liver microsomes in the presence of a NADPH-generating system and the formation of desmethylnaproxen was measured by high-performance liquid chromatography (HPLC). To further clarify the specific isoforms involved, experiments were conducted with preparations expressing only a single P450 isoform (vaccinia virus-expressed cells and microsomes derived from a lymphoblastoid cell line, each transfected with specific P450 cDNAs) as well as inhibition studies using human liver microsomes and putative specific P450 inhibitors.
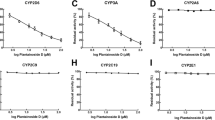
Results: In human liver microsomes (n=7), desmethylnaproxen formation was observed with a mean kM of 92 (21) μmol · l−1, Vmax of 538 pmol · min−1 · mg−1 protein and Cint2 (reflective of a second binding site) of 0.36 μl · min−1 · mg−1 protein. This Cint2 term was added since Eadie-Scatchard analysis suggested the involvement of more than one enzyme. Studies using putative specific P450 inhibitors demonstrated inhibition of this␣reaction by sulfaphenazole, (apparent Ki= 1.6 μmol · l−1), warfarin (apparent Ki=27 μmol · l−1), piroxicam (apparent Ki=23 μmol · l−1) and tolbutamide (apparent Ki=128 μmol · l−1). No effect was observed when α-naphthoflavone and troleandomycin were employed as inhibitors, but reaction with furafylline produced, on average, a maximum inhibition of 23%. At a naproxen concentration of 150 μmol · l−1, formation of desmethylnaproxen was observed in cells expressing P450 1A2, 2C8, 2C9 and its allelic variant 2C9R144C. To further characterize these reactions, saturation kinetics experiments were conducted for the P450s 1A2, 2C8 and 2C9. The kM and Vmax for P450 1A2 were 189.5 μmol · l−1 and 7.3 pmol · min−1 · pmol−1 P450, respectively. Likewise, estimates of kM and Vmax for P450 2C9 were 340.5 μmol · l−1 and 41.4 pmol · min−1 · pmol−1 P450, respectively. Reliable estimates of kM and Vmax could not be made for P450 2C8 due to the nonsaturable nature of the process over the concentration range studied.
Conclusion: Multiple cytochrome P450 isoforms (P450 1A2, 2C8 and 2C9) appear to be involved in naproxen demethylation, although 2C9 appears to be the predominant form.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 16 September 1996 / Accepted in revised form: 20 December 1996
Rights and permissions
About this article
Cite this article
Tracy, T., Marra, C., Wrighton, S. et al. Involvement of multiple cytochrome P450 isoforms in naproxen O-demethylation. E J Clin Pharmacol 52, 293–298 (1997). https://doi.org/10.1007/s002280050293
Issue Date:
DOI: https://doi.org/10.1007/s002280050293