Abstract
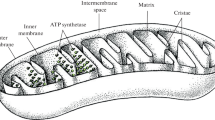
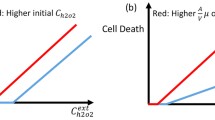
Cationic lipophilic dyes can accumulate in mitochondria, and especially in mitochondria of tumor cells. We investigated the chemical properties and the processes allowing selective uptake into tumor cells using the Fick–Nernst–Planck equation. The model simulates uptake into cytoplasm and mitochondria and is valid for neutral molecules and ions, and thus also for weak electrolytes. The differential equation system was analytically solved for the steady-state and the dynamic case. The parameterization was for a generic human cell, with a 60 mV more negative potential at the inner mitochondrial membrane of generic tumor cells. The chemical input data were the lipophilicity (logKOW), the acid/base dissociation constant (pKa) and the electric charge (z). Accumulation in mitochondria occurred for polar acids with pKa between 5 and 9 owing to the ion trap, and for lipophilic bases with pKa>11 or permanent cations owing to electrical attraction. Selective accumulation in tumor cells was found for monovalent cations or strong bases with logKOW of the cation between −2 and 2, with the optimum near 0. The results are in agreement with experimental results for rhodamine 123, a series of cationic triarylmethane dyes, F16 and MKT-077, an anticancer drug targeting tumor mitochondria.







Similar content being viewed by others
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the cell, 4th ed. Garland, Abingdon, UK
Appelo CAJ, Postma D (1999) Geochemistry and groundwater pollution, 4th ed. Balkema, Rotterdam, NL
Briggs GE, Hope AB, Robertson RN (1961) Electrolytes and plant cells. In: James WO (ed) Botanical monographs, Vol. 1. Blackwell, Oxford, UK
Briggs GG, Rigitano RLO, Bromilow RH (1987) Physico-chemical factors affecting uptake by roots and translocation to shoots of weak acids in barley. Pestic Sci 19:101–112
Britten CD, Rowinsky EK, Baker SD, Weiss GR, Smith L, Stephenson J, Rothenberg M, Smetzer L, Cramer J, Collins W, Von Hoff DD, Eckhardt SG (2000) A phase I and pharmacokinetic study of the mitochondrial-specific rhodacyanine dye analog MKT 077. Clin Cancer Res 6:42–49
Chen LB (1988) Mitochondrial membrane potential in living cells. Ann Rev Cell Biol 4:155–181
Chen LB (1989) Fluorescent labeling of mitochondria. Methods of cell biology, Vol 29. Academic, New York, USA, pp 103–123
Davis S, Weiss MJ, Wong JR, Lampidis TJ, Chen LB (1985). Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Biol Chem, 260:13844–13850
Eytan GD, Regev R, Hurwitz CD, Assaraf YG (1997) Efficiency of P-glycoprotein-mediated exclusion of rhodamin dyes from multidrug-resistant cells is determined by their passive transmembrane movement rate. Eur J Biochem 248:104–112
Fantin VR, Beradi MJ, Scorrano L, Korsmeyer SJ, Leder P (2002) A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2:29–41
Ferguson MW, Beaumont PC, Jones SE, Navaratman S, Parsons BJ (1999). Excited state and free radical properties of Rhodamine 123: a laser flash photolysis and radiolysis study. Phys Chem Chem Phys 1:261–268
Grayson BT, Kleier DA (1990). Phloem mobility of xenobiotics. IV. Modelling of pesticide movement in plants. Pestic Sci 30:67–69
Hansch C, Leo A (1995) Exploring QSAR: fundamentals and applications in chemistry and biology. American Chemical Society, Washington DC, USA.
Henderson LJ (1908). Concerning the relationship between the strength of acids and their capacity to preserve neutrality. J Physiol 21:173–179
Horobin RW (2001). Uptake, distribution, and accumulation of dyes and fluorescent probes withing living cells: a structure-activity modelling approach. Adv Colour Sci Technol 4:101–107
Horobin RW, Kiernan JA, (eds) (2002). Conn’s biological stains: handbook of dyes, stains and fluorochromes for use in biology and medicine. BIOS Scientific Publishers, Oxford, UK, pp 170–171
Hsu FC, Kleier DA (1996). Phloem mobility of xenobiotics VII. The design of phloem systemic pesticides. Weed Sci 44:749–756
Hunziker RW, Escher BI, Schwarzenbach RP (2001) pH dependency of the partitioning of triphenyltin and tributyltin between phosphatidilcholine liposomes and water. Environ Sci Technol 35:3899–3904
Inoue J, Chamberlain K, Bromilow RH (1998). Physico-chemical factors affecting the uptake by roots and translocation to shoots of amine bases in barley. Pestic Sci 54:8–21
Kandela IK, Bartlett JA, Indig GL (2002). Effect of molecular structure on the selective phototoxicity of triarylmethane dyes towards tumor cells. Photochem Photobiol Sci 1:309–314
Kandela IK, Lee W, Indig GL (2003). Effect of the lipophilic/hydrophilic character of cationic triarylmethane dyes on their selective phototoxicity toward tumor cells. Biotechnic Histochem 78:157–189
Kleier DA (1988). Phloem mobility of xenobiotics I. Mathematical model unifying the weak acid and intermediate permeability theories. Plant Physiol 86:803–810
Kuchling H (1981) Taschenbuch der Physik, 3rd ed, Harri Deutsch, Thun and Frankfurt am Main, D
Modica-Napolitano JS, Aprille JR (2001) Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliver Rev 49:63–70
Nazaroff WN, Alvarez-Cohen L (2001) Environmental engineering science. Wiley, New York, USA. pp 624–625
Nernst W (1889). Die elektrische Wirksamkeit der Jonen. Z Physik Chem 4:129–181
Rashid F, Horobin RW (1991). Accumulation of fluorescent non-cationic probes in mitochondria of cultured cells: observations, a proposed mechanism and some implications. J Microsc 163:233–241
Raven JA (1975). Transport of indolacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol 74:163–172
Riederer M (1995) Partitioning and transport of organic chemicals between the atmospheric environment and leaves. In: Trapp S, Mc Farlane JC (eds) Plant contamination. Modeling and simulation of organic chemicals processes. Lewis, Boca Raton, FL, USA, pp 153–190
Schönherr J, Riederer M (1989) Foliar penetration and accumulation of organic chemicals in plant cuticles. Reviews Environ Contam Toxicol 108:1–70
Tatsuta N, Suzuki N, Mochizuki T, Koya K, Kawakami M, Shishido T, Motoji N, Kuroiwa H, Shigemaatsu A, Chen LB (1999) Pharmacokinetic analysis and antitumor efficiency of MKT-077, a novel antitumor agent. Cancer Chemother Pharmacol 43:295–301
Trapp S (2000) Modeling uptake into roots and subsequent translocation of neutral and ionisable organic compounds. Pest Manage Sci 56:767–778
Trapp S (2004) Plant Uptake and Transport Models for Neutral and Ionic Chemicals. Environ Sci Poll Res 11:33–39 doi: espr2003.08.169
Trapp S, Matthies M (1995) Generic one-compartment model for uptake of organic chemicals by foliar vegetation. Environ Sci Technol 29:2333–2338; erratum 30:360
Acknowledgements
S.T. appreciates Hans Mosbæk for hints in chemistry, Michael Matthies for advice in systems science and Jörg Schönherr for scientific discussion. R.W.H. thanks I. McGrath, Division of Neuroscience & Biomedical Systems, University of Glasgow, for provision of facilities. Thanks to Birte Brejl for preparing the graphs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trapp, S., Horobin, R.W. A predictive model for the selective accumulation of chemicals in tumor cells. Eur Biophys J 34, 959–966 (2005). https://doi.org/10.1007/s00249-005-0472-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-005-0472-1