Abstract
Introduction
Overexpression of epidermal growth factor receptor (EGFR) is a prognostic and predictive biomarker in a number of malignant tumours. Radionuclide molecular imaging of EGFR expression in cancer could influence patient management. However, EGFR expression in normal tissues might complicate in vivo imaging. The aim of this study was to evaluate if optimization of the injected protein dose might improve imaging of EGFR expression in tumours using a novel EGFR-targeting protein, the DOTA-ZEGFR:2377 Affibody molecule.
Methods
An anti-EGFR Affibody molecule, ZEGFR:2377, was labelled with 111In via the DOTA chelator site-specifically conjugated to a C-terminal cysteine. The affinity of DOTA-ZEGFR:2377 for murine and human EGFR was measured by surface plasmon resonance. The cellular processing of 111In-DOTA-ZEGFR:2377 was evaluated in vitro. The biodistribution of radiolabelled Affibody molecules injected in a broad range of injected Affibody protein doses was evaluated in mice bearing EGFR-expressing A431 xenografts.
Results
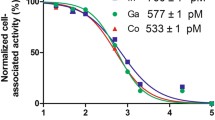
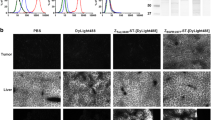
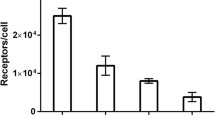
Site-specific coupling of DOTA provided a uniform conjugate possessing equal affinity for human and murine EGFR. The internalization of 111In-DOTA-ZEGFR:2377 by A431 cells was slow. In vivo, the conjugate accumulated specifically in xenografts and in EGFR-expressing tissues. The curve representing the dependence of tumour uptake on the injected Affibody protein dose was bell-shaped. The highest specific radioactivity (lowest injected protein dose) provided a suboptimal tumour-to-blood ratio. The results of the biodistribution study were confirmed by γ-camera imaging.
Conclusion
The 111In-DOTA-ZEGFR:2377 Affibody molecule is a promising tracer for radionuclide molecular imaging of EGFR expression in malignant tumours. Careful optimization of protein dose is required for high-contrast imaging of EGFR expression in vivo.





Similar content being viewed by others
References
Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8.
Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32.
Zlobec I, Vuong T, Hayashi S, Haegert D, Tornillo L, Terracciano L, et al. A simple and reproducible scoring system for EGFR in colorectal cancer: application to prognosis and prediction of response to preoperative brachytherapy. Br J Cancer. 2007;96:793–800.
Nieto Y, Nawaz F, Jones RB, Shpall EJ, Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J Clin Oncol. 2007;25:4405–13.
Schlomm T, Kirstein P, Iwers L, Daniel B, Steuber T, Walz J, et al. Clinical significance of epidermal growth factor receptor protein overexpression and gene copy number gains in prostate cancer. Clin Cancer Res. 2007;13:6579–84.
Psyrri A, Kassar M, Yu Z, Bamias A, Weinberger PM, Markakis S, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–43.
Kersting C, Packeisen J, Leidinger B, Brandt B, von Wasielewski R, Winkelmann W, et al. Pitfalls in immunohistochemical assessment of EGFR expression in soft tissue sarcomas. J Clin Pathol. 2006;59:585–90.
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–6.
Bentzen SM, Atasoy BM, Daley FM, Dische S, Richman PI, Saunders MI, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23:5560–7.
Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, et al. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol. 2005;74:101–8.
Giltnane JM, Rydén L, Cregger M, Bendahl PO, Jirström K, Rimm DL. Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J Clin Oncol. 2007;25:3007–14.
Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004;22:4772–8.
Choong LY, Lim S, Loh MC, Man X, Chen Y, Toy W, et al. Progressive loss of epidermal growth factor receptor in a subpopulation of breast cancers: implications in target-directed therapeutics. Mol Cancer Ther. 2007;6:2828–42.
Pantaleo MA, Nannini M, Maleddu A, Fanti S, Nanni C, Boschi S, et al. Experimental results and related clinical implications of PET detection of epidermal growth factor receptor (EGFr) in cancer. Ann Oncol. 2009;20:213–26.
Gelovani JG. Molecular imaging of epidermal growth factor receptor expression-activity at the kinase level in tumors with positron emission tomography. Cancer Metastasis Rev. 2008;27:645–53.
Mishani E, Abourbeh G, Eiblmaier M, Anderson CJ. Imaging of EGFR and EGFR tyrosine kinase overexpression in tumors by nuclear medicine modalities. Curr Pharm Des. 2008;14:2983–98.
Goldenberg A, Masui H, Divgi C, Kamrath H, Pentlow K, Mendelsohn J. Imaging of human tumor xenografts with an indium-111-labeled anti-epidermal growth factor receptor monoclonal antibody. J Natl Cancer Inst. 1989;81:1616–25.
Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991;83:97–104.
Reilly RM, Kiarash R, Sandhu J, Lee YW, Cameron RG, Hendler A, et al. A comparison of EGF and MAb 528 labeled with 111In for imaging human breast cancer. J Nucl Med. 2000;41:903–11.
Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850–8.
Milenic DE, Wong KJ, Baidoo KE, Ray GL, Garmestani K, Williams M, et al. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm. 2008;23:619–31.
Ping Li W, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother Radiopharm. 2008;23:158–71.
Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–31.
Cuartero-Plaza A, Martínez-Miralles E, Rosell R, Vadell-Nadal C, Farré M, Real FX. Radiolocalization of squamous lung carcinoma with 131I-labeled epidermal growth factor. Clin Cancer Res. 1996;2:13–20.
Rusckowski M, Qu T, Chang F, Hnatowich DJ. Technetium-99m labeled epidermal growth factor-tumor imaging in mice. J Pept Res. 1997;50:393–401.
Capala J, Barth RF, Bailey MQ, Fenstermaker RA, Marek MJ, Rhodes BA. Radiolabeling of epidermal growth factor with 99mTc and in vivo localization following intracerebral injection into normal and glioma-bearing rats. Bioconjug Chem. 1997;8:289–95.
Sundberg AL, Orlova A, Bruskin A, Gedda L, Carlsson J, Blomquist E, et al. [(111)In]Bz-DTPA-hEGF: preparation and in vitro characterization of a potential anti-glioblastoma targeting agent. Cancer Biother Radiopharm. 2003;18(4):643–54.
Babaei MH, Almqvist Y, Orlova A, Shafii M, Kairemo K, Tolmachev V. [99mTc]HYNIC-hEGF, a potential agent for imaging of EGF receptors in vivo: preparation and pre-clinical evaluation. Oncol Rep. 2005;13:1169–75.
Velikyan I, Sundberg AL, Lindhe O, Höglund AU, Eriksson O, Werner E, et al. Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med. 2005;46(11):1881–8.
Nygren PA. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008;275:2668–76.
Nilsson FY, Tolmachev V. Affibody molecules: new protein domains for molecular imaging and targeted tumor therapy. Curr Opin Drug Discov Devel. 2007;10:167–75.
Orlova A, Feldwisch J, Abrahmsén L, Tolmachev V. Update: affibody molecules for molecular imaging and therapy for cancer. Cancer Biother Radiopharm. 2007;22:573–84.
Nordberg E, Orlova A, Friedman M, Tolmachev V, Ståhl S, Nilsson FY, et al. In vivo and in vitro uptake of 111In, delivered with the affibody molecule (ZEGFR:955)2, in EGFR expressing tumour cells. Oncol Rep. 2008;19:853–7.
Friedman M, Orlova A, Johansson E, Eriksson TL, Höidén-Guthenberg I, Tolmachev V, et al. Directed evolution to low nanomolar affinity of a tumor-targeting epidermal growth factor receptor-binding affibody molecule. J Mol Biol. 2008;376:1388–402.
Tolmachev V, Friedman M, Sandström M, Eriksson TL, Rosik D, Hodik M, et al. Affibody molecules for epidermal growth factor receptor targeting in vivo: aspects of dimerization and labeling chemistry. J Nucl Med. 2009;50:274–83.
Mume E, Orlova A, Larsson B, Nilsson AS, Nilsson FY, Sjöberg S, et al. Evaluation of ((4-hydroxyphenyl)ethyl)maleimide for site-specific radiobromination of anti-HER2 affibody. Bioconjug Chem. 2005;16:1547–55.
Ahlgren S, Orlova A, Rosik D, Sandström M, Sjöberg A, Baastrup B, et al. Evaluation of maleimide derivative of DOTA for site-specific labeling of recombinant affibody molecules. Bioconjug Chem. 2008;19:235–43.
Tolmachev V, Xu H, Wållberg H, Ahlgren S, Hjertman M, Sjöberg A, et al. Evaluation of a maleimido derivative of CHX-A'' DTPA for site-specific labeling of affibody molecules. Bioconjug Chem. 2008;19:1579–87.
Tolmachev V, Orlova A, Wei Q, Bruskin A, Carlsson J, Gedda L. Comparative biodistribution of potential anti-glioblastoma conjugates [111In]DTPA-hEGF and [111In]Bz-DTPA-hEGF in normal mice. Cancer Biother Radiopharm. 2004;19:491–501.
Wållberg H, Orlova A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: implications for development of labeled tracers. Cancer Biother Radiopharm. 2008;23:435–42.
Maecke HR, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. J Nucl Med. 2005;46(Suppl 1):172S–8S.
Biddlecombe GB, Rogers BE, de Visser M, Parry JJ, de Jong M, Erion JL, et al. Molecular imaging of gastrin-releasing peptide receptor-positive tumors in mice using 64Cu- and 86Y-DOTA-(Pro1,Tyr4)-bombesin(1-14). Bioconjug Chem. 2007;18:724–30.
Wållberg H, Ahlgren S, Widström C, Orlova A. Evaluation of the radiocobalt-labeled [MMA-DOTA-Cys61]-ZHER2:2395-Cys Affibody molecule for targeting of HER2-expressing tumors. Mol Imaging Biol 2009. doi:10.1007/s11307-009-0238-8.
ImClone Systems Incorporated. Cetuximab: epidermal growth factor receptor (EGFR) antibody, version 9.0. ImClone Investigator Brochure. New York: ImClone Systems, 2003.
Gainkam LO, Huang L, Caveliers V, Keyaerts M, Hernot S, Vaneycken I, et al. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J Nucl Med. 2008;49:788–95.
de Jong M, Breeman WA, Bernard BF, et al. Tumour uptake of the radiolabelled somatostatin analogue [DOTA0,TYR3]octreotide is dependent on the peptide amount. Eur J Nucl Med. 1999;26:693–8.
Schuhmacher J, Zhang H, Doll J, Mäcke HR, Matys R, Hauser H, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6-14) analog. J Nucl Med. 2005;46:691–9.
Acknowledgments
This study was supported by grants from the Swedish Cancer Society (Cancerfonden) and the Swedish Research Council (Vetenskapsrådet). We thank Veronika Eriksson and the staff of the animal facility at Rudbeck Laboratory for technical assistance.
Disclosures
The authors, Orlova Anna, Helena Wållberg and Vladimir Tolmachev had earlier, and Daniel Rosik, Anna Sjöberg, Monika Hansson, Anders Wennborg have currently an affiliation (employment) with Affibody AB, Bromma, Sweden, which holds the intellectual property rights and trademarks for Affibody molecules.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolmachev, V., Rosik, D., Wållberg, H. et al. Imaging of EGFR expression in murine xenografts using site-specifically labelled anti-EGFR 111In-DOTA-ZEGFR:2377 Affibody molecule: aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging 37, 613–622 (2010). https://doi.org/10.1007/s00259-009-1283-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1283-x