Abstract
Purpose
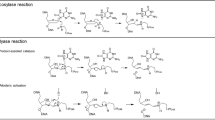
To investigate the interaction of the electrophilic species generated by the decomposition of the antineoplastic prodrug 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine (VNP40101M) on the ability of O6-alkylguanine-DNA alkyltransferase (AGT) to repair alkylated O6-chloroethylguanine and/or N1,O6-ethanoguanine DNA lesions.
Materials and methods
The contributions of inhibitory electrophilic species generated from VNP40101M towards AGT was assessed using analogues that selectively generated either the chloroethylating or the carbamoylating components of VNP40101M. The activity of AGT was determined from the inhibition of crosslink formation from O6-chloroethylguanine and/or N1,O6-ethanoguanine lesions. The half-lives of sulfonylhydrazine derivatives and isocyanates were measured using an acidification assay which gives a change in absorbance proportional to the release or consumption of small quantities of protons.
Results
Both of the reactive components produced by VNP40101M directly inactivated cloned human AGT; the carbamoylating moiety (IC50 about 13 μM) was approximately seven- to eight-fold more potent than the alkylating component(s) (IC50 about 100 μM). These inhibitory actions were moderated by the addition of naked T5 bacteriophage DNA. Thus, AGT bound to DNA was markedly more resistant than free AGT to these electrophilic species. DNA also blocked the spontaneous loss of AGT activity which occurred upon incubation of this protein under mild conditions.
Conclusions
The reaction of AGT with the methyl isocyanate generated from the decomposition of VNP40101M increased the net number of crosslinks generated by VNP40101M compared to a sulfonylhydrazine prodrug that formed the equivalent alkylating species in the absence of the cogeneration of methyl isocyanate. These actions may be of significance to the antineoplastic activity of VNP40101M.




Similar content being viewed by others
Notes
Ten μl of 100 μg/ml of T5 DNA = 1 μg DNA; T5 DNA Mr = 68,000,000 = 15 fmol DNA; 30–40% crosslinking = 4.5–6.0 fmol of crosslinks generated by 2 nmol 90CE (10 μl of 0.2 mM). Moles of crosslinks/mole alkylator = crosslink molar yield = 0.025%
References
Shyam K, Penketh PG, Loomis RH, Rose WC, Sartorelli AC (1996) Antitumor 2-(aminocarbonyl)-1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazines. J Med Chem 39:796
Finch RA, Shyam K, Penketh PG, Sartorelli AC (2001) 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylamino)carbonylhydrazine (101M): a novel sulfonylhydrazine prodrug with broad-spectrum antineoplastic activity. Cancer Res 61:3033
Penketh PG, Shyam K, Sartorelli AC (2000) Comparison of DNA lesions produced by tumor-inhibitory 1,2-bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochem Pharmacol 59:283
Penketh PG, Shyam K, Sartorelli AC (1994) Studies on the mechanism of decomposition and structural factors affecting the aqueous stability of 1,2-bis(sulfonyl)-1-alkylhydrazines. J Med Chem 37:2912
Pearson PG, Slatter JG, Rashed MS, Han DH, Baillie TA (1991) Carbamoylation of peptides and proteins in vitro by S-(N-methylcarbamoyl)glutathione and S-(N-methylcarbamoyl)cysteine, two electrophilic S-linked conjugates of methyl isocyanate. Chem Res Toxicol 4:436
Johnston TP, Montgomery JA (1986) Relationship of structure to anticancer activity and toxicity of the nitrosoureas in animal systems. Cancer Treat Rep 70:13
Baumann RP, Shyam K, Penketh PG, Remack J, Brent TP, Sartorelli AC (2003) 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine (VNP40101M): II. Role of O6-alkylguanine DNA alkyltransferase in cytotoxicity. Cancer Chemother Pharmacol 53 (companion paper) DOI 10.1007/s00280-003-0739-0
Babson JR, Reed DJ (1978) Inactivation of glutathione reductase by 2-chloroethyl nitrosourea-derived isocyanates. Biochem Biophys Res Commun 83:754
Frischer H, Ahmad T (1977) Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med 89:1080
Karplus PA, Krauth-Siegel RL, Schirmer RH, Schulz GE (1988) Inhibition of human glutathione reductase by the nitrosourea drugs 1,3-bis(2-chloroethyl)-1-nitrosourea and 1-(2-chloroethyl)-3-(2-hydroxyethyl)-1-nitrosourea. A crystallographic analysis. Eur J Biochem 171:193
Bodell WJ, Tokuda K, Ludlum DB (1988) Differences in DNA alkylation products formed in sensitive and resistant human glioma cells treated with N-(2-chloroethyl)-N-nitrosourea. Cancer Res 48:4489
Pegg AE, Dolan ME, Moschel RC (1995) Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. In: Cohn WE, Moldave K (eds) Progress in nucleic acid research and molecular biology, vol 51. Academic Press, San Diego, p 167
Ludlum DB (1997) The chloroethylnitrosoureas: sensitivity and resistance to cancer chemotherapy at the molecular level. Cancer Invest 15:588
Parker S, Kirk MC, Ludlum DB (1987) Synthesis and characterization of O6-(2-chloroethyl)guanine: a putative intermediate in the cytotoxic reaction of chloroethylnitrosoureas with DNA. Biochem Biophys Res Commun 148:1124
Brent TP (1985) Isolation and purification of O6-alkylguanine-DNA alkyltransferase from human leukemic cells. Prevention of chloroethylnitrosourea-induced crosslinks by purified enzyme. Pharmacol Ther 31:121
Gonzaga PE, Harris L, Margison GP, Brent TP (1990) Evidence that covalent complex formation between BCNU-treated oligonucleotides and E. coli alkyltransferases requires the O6-alkylguanine function. Nucleic Acids Res 18:3961
Potter PM, Lassiter AL, Brent TP (1993) Purification of a human DNA repair protein using an alternative to DNA-affinity chromatography. Methods Mol Cell Biol 4:139
Penketh PG, Shyam K, Sartorelli AC (1997) Fluorometric assay for the determination of DNA-DNA crosslinks utilizing Hoechst 33258 at neutral pH values. Anal Biochem 252:210
Penketh PG, Shyam K, Patton CL, Sartorelli AC (1996) Spectrophotometric assay for processes involving changes in hydrogen ion concentration in aqueous solution. Anal Biochem 238:46
Ali-Osman F (1989) Quenching of DNA crosslink precursors of chloroethylnitrosoureas and attenuation of DNA interstrand crosslinking by glutathione. Cancer Res 49:5258
Hilton J, Maldarelli F, Sargent S (1978) Evaluation of the role of isocyanates in the action of therapeutic nitrosoureas. Biochem Pharmacol 27:1359
Pegg AE (2000) Repair of O6-alkylguanine by alkyltransferases. Mutat Res 463:83
Loktionova NA, Pegg AE (2002) Interaction of mammalian O6-alkylguanine-DNA alkyltransferase with O6-benzylguanine. Biochem Pharmacol 63:1431
Belanich M, Randal T, Pastor MA, Kibitel JT, Alas LG, Dolan ME, Schold SC, Gander M, Lejeune FJ, Li BFL, White AB, Wasserman P, Citron ML, Yarosh DB (1996) Intracellular localization and intercellular heterogeneity of the human DNA repair protein O(6)-methylguanine-DNA methyltransferase. Cancer Chemother Pharmacol 37:547
Maker HS, Weiss C, Brannan TS (1983) The effects of BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea) and CCNU (1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea) on glutathione reductase and other enzymes in mouse tissue. Res Commun Chem 40:355
Boutin TA, Norbeck K, Moldeus P, Genton A, Pariare M, Bizzari JP, Lavielle G, Cudennec CA (1989) Effects of the new nitrosourea derivative, fotemustine, on the glutathione reductase activity in rat tissues in vivo and in isolated rat hepatocytes. Eur J Cancer Clin Oncol 25:1311
Kann HE, Kohn WK, Lyles JM (1974) Inhibition of DNA repair by the 1,3-bis(2-chloroethyl)-1-nitrosourea breakdown product, 2-chloroethyl isocyanate. Cancer Res 34:398
Baril BB, Baril EF, Laszlo J, Wheeler GP (1975) Inhibition of rat liver DNA polymerase by nitrosoureas and isocyanates. Cancer Res 35:1
Petak K, Mihalik R, Bauer PI, Suli-Vargha H, Sebestyen A, Kopper L (1988) BCNU is a caspase-mediated inhibitor of drug-induced apoptosis. Cancer Res 58:614
Slatter JG, Rashed MS, Pearson PG, Han DH, Baillie, TA (1991) Biotransformation of methyl isocyanate in the rat. Evidence for glutathione conjugation as a major pathway of metabolism and implications for isocyanate-mediated toxicities. Chem Res Toxicol 4:157
Glassner BJ, Weeda G, Allan JM, Broekhof JLM, Carls NHE, Donker I, Engelward BP, Hampson RJ, Hermus R, Hickman MJ, Roth RB, Warren HB, Wu MM, Hoeijmakers JHJ, Samson LD (1999) DNA repair methyltransferase (Mgmt) knockout mice are sensitive to the lethal effects of chemotherapeutic alkylating agents. Mutagenesis 14:339
Kaina B, Fritz G, Mitra S, Coquerelle T (1991) Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis 12:1857
Schallreuter KU, Gleason FK, Wood JM (1990) The mechanism of action of the nitrosourea anti-tumor drugs on thioredoxin reductase, glutathione reductase and ribonucleotide reductase. Biochim Biophys Acta 1054:14
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by U.S. Public Health Service Grants CA-90671 (A.C.S.) and CA-14799 (T.P.B.) from the National Cancer Institute.
Rights and permissions
About this article
Cite this article
Penketh, P.G., Shyam, K., Baumann, R.P. et al. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[(methylamino)carbonyl]hydrazine (VNP40101M): I. Direct inhibition of O6-alkylguanine-DNA alkyltransferase (AGT) by electrophilic species generated by decomposition. Cancer Chemother Pharmacol 53, 279–287 (2004). https://doi.org/10.1007/s00280-003-0740-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0740-7