Abstract
Purpose
The aim of this study was to investigate the transport mechanisms of transporters that contribute to the intestinal uptake of 7-ethyl-10-hydroxycamptothecin (SN-38).
Methods
Human intestinal epithelial Caco-2 cells were used to investigate the mechanistic basis of transepithelial uptake of SN-38. We investigated the characteristics of SN-38 uptake into Caco-2 cells. The effects of baicalin and sulfobromophthalein (BSP) on the uptake of SN-38 by Caco-2 cells were examined.
Results
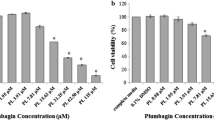
Uptake of SN-38 was significantly reduced at 4°C. Baicalin inhibited the uptake of SN-38 in a concentration-dependent manner. BSP significantly reduced the uptake of SN-38. However, probenecid, pravastatin and grepafloxacin did not affect the uptake of SN-38.
Conclusions
The results suggest that a specific transport system mediates the uptake of SN-38 across the apical membrane in Caco-2 cells.


Similar content being viewed by others
References
Abigerges D, Armand JP, Chabot GG, Da Costa L, Fadel E, Cote C, Herait P, Gandia D (1994) Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst 86:446–449
Akimoto K, Kawai A, Ohya K (1994) Kinetic studies of the hydrolysis and lactonization of camptothecin and its derivatives, CPT-11 and SN-38, in aqueous solution. Chem Pharm Bull 42:2135–2138
Atsumi R, Suzuki W, Hakusui H (1991) Identification of the metabolites of irinotecan, a new derivative of camptothecin, in rat bile and its biliary excretion. Xenobiotica 21:1159–1169
Enerson BE, Drewes LR (2003) Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci 92:1531–1544
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Horikawa M, Kato Y, Sugiyama Y (2002) Reduced gastrointestinal toxicity following inhibition of the biliary excretion of irinotecan and its metabolites by probenecid in rats. Pharm Res 19:1345–1353
Houghton PJ, Cheshire PJ, Hallman JC, Bissery MS, Mathieu-Boue A, Houghton JA (1993) Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res 53:2823–2829
Itagaki S, Sugawara M, Kobayashi M, Miyazaki K, Iseki K (2003) Mechanism of active secretion of phenolsulfonphthalein in the liver via MRP2 (abcc2), an organic anion transporter. Drug Metab Pharmacokinet 18:238–244
Itoh T, Itagaki S, Sasaki K, Hirano T, Takemoto I, Iseki K (2004) Pharmacokinetic modulation of irinotecan metabolites by sulfobromophthalein. J Pharm Pharmacol 56:809–812
Itoh T, Takemoto I, Itagaki S, Sasaki K, Hirano T, Iseki K (2004) Biliary excretion of irinotecan and its metabolites. J Pharm Pharm Sci 7:13–18
Katsura T, Inui KI (2003) Intestinal absorption of drugs mediated by drug transporters: mechanisms and regulation. Drug Metab Pharmacokinet 18:1–15
Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mizuno N, Sugiyama Y (2002) Drug transporters: their role and importance in the selection and development of new drugs. Drug Metab Pharmacokinet 17:93–108
Narita M, Nagai E, Hagiwara H, Aburada M, Yokoi T, Kamataki T (1993) Inhibition of beta-glucuronidase by natural glucuronides of kampo medicines using glucuronide of SN-38 (7-ethyl-10-hydroxycamptothecin) as a substrate. Xenobiotica 23:5–10
Rivory LP, Bowles MR, Robert J, Pond SM (1996) Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochem Pharmacol 52:1103–1111
Sakata Y, Suzuki H, Kamataki T (1994) Preventive effect of TJ-14, a kampo (Chinese herb) medicine, on diarrhea induced by irinotecan hydrochloride (CPT-11). Jpn J Cancer Chemother 21:1241–1244
Satoh H, Ishikawa H, Murakami O, Yamashita Y, Maeno T, Kamma H, Ohtsuka M, Hasegawa S (1999) Reduction by hange-shashin-to (TJ-14), a herbal medicine on gastrointestinal tract complications induced by CPT-11 containing chemotherapy. Cancer Res Ther Control 7:321–323
Takasuna K, Kasai Y, Kitano Y, Mori K, Kobayashi R, Hagiwara T, Kakihata K, Hirohashi M, Nomura M, Nagai E, Kamataki T (1995) Prospective effect of Kampo medicines and baicalin against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn J Cancer Res 86:978–984
Takasuna K, Hagiwara T, Hirohashi M, Nomura M, Nagai E, Yokoi T, Kamataki T (1996) Involvement of b-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56:3752–3757
Tamai I, Takanaga H, Maeda H, Sai Y, Ogihara T, Higashida H, Tsuji A (1995) Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun 214:482–489
Tsuji A (2002) Transporter-mediated drug interactions. Drug Metab Pharmacokinet 17:253–274
Yamaguchi H, Yano I, Saito H, Inui K (2001) Transport characteristics of grepafloxacin and levofloxacin in the human intestinal cell line Caco-2. Eur J Pharmacol 431:297–303
Acknowledgements
We thank Dr. S. Miyauchi for his advice on the experimental technique. This work was supported in part by grants from the Akiyama Foundation and the Japan Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itoh, T., Itagaki, S., Sumi, Y. et al. Uptake of irinotecan metabolite SN-38 by the human intestinal cell line Caco-2. Cancer Chemother Pharmacol 55, 420–424 (2005). https://doi.org/10.1007/s00280-004-0937-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0937-4