Abstract
Purpose
To evaluate the ability of a physiology-based pharmacokinetic (PBPK) model to predict the systemic drug exposure of high- and low-dose etoposide in children from a model developed with adult data.
Methods
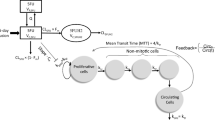
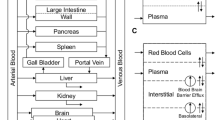
Simulations were performed with PK-Sim® (Bayer Technology Services). Model development was done using data from adult patients receiving etoposide in a conventional and high-dose polychemotherapy regimen before stem cell transplantation. Michaelis–Menten parameters from in vitro experiments reported in the literature were applied to describe the metabolism and excretion processes by P450 enzymes and transporters. The model was scaled down to children and compared to etoposide plasma concentrations in this age group.
Results
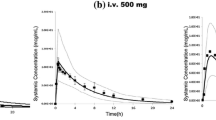
Simulated plasma concentration–time courses of protein-bound and free etoposide in adults for high- and low-dose schedules agreed with the observed data. Mean simulated total clearance of high- and low-dose etoposide was 0.70 ml/min/kg (Clobserved: 0.70 ml/min/kg) versus 0.50 ml/min/kg (Clobserved: 0.60 ml/min/kg), respectively. Integrated Michaelis–Menten kinetics was adequately transformed to age-related pharmacokinetics in children. Predictions of the pharmacokinetics in different age groups were also in good agreement with observed data. Drug interactions triggered by P-glycoprotein inhibitors or nephrotoxic drugs can also be elucidated.
Conclusions
The PBPK model matched the pharmacokinetics in different dosing regimens in adults. Furthermore, the scaling procedure from the adult model to children provides useful predictions for paediatric patients. Comedication with drugs influencing the metabolism and excretion has to be taken into account. This approach could be useful for planning pharmacokinetic studies in children.




Similar content being viewed by others
References
Palle J, Frost BM, Gustafsson G, Hellebostad M, Kanerva J, Liliemark E, Schmiegelow K, Lönnerholm G (2006) Etoposide pharmacokinetics in children treated for acute myeloid leukemia. Anticancer Drugs 17:1087–1094
Relling MV, McLeod HL, Bowman LC, Santana VM (1994) Etoposide pharmacokinetics and pharmacodynamics after acute and chronic exposure to cisplatin. Clin Pharmacol Ther 56:503–511
Panetta JC, Wilkinson M, Pui CH, Relling MV (2002) Limited and optimal sampling strategies for etoposide and etoposide catechol in children with leukaemia. J Pharmacokin Pharmacodyn 29:171–188
Urien S, Doz F, Giraud C, Rey E, Gentet JC, Chastagner P, Vassal G, Corradini N, Auvrignon A, Leblond P, Rubie H, Treluyer JM (2011) Developmental pharmacokinetics of etoposide in 67 children: lack of dexamethasone effect. Cancer Chemother Pharmacol 67:597–603
Veal GJ, Cole M, Errington J, Pearson AD, Gerrard M, Whyman G, Ellershaw C, Boddy AV (2010) Pharmacokinetics of carboplatin and etoposide in infant neuroblastoma patients. Cancer Chemother Pharmacol 65:1057–1066
Würthwein G, Klingebiel T, Krümpelmann S, Metz M, Schwenker K, Kranz K, Lanvers C, Boos J (2002) Population pharmacokinetics of high-dose etoposide in children receiving different conditioning regimens. Anticancer Drugs 13:101–110
Würthwein G, Krümpelmann S, Tillmann B, Real E, Schulze-Westhoff P, Jürgens H, Boos J (1999) Population pharmacokinetic approach to compare oral and i.v. administration of etoposide. Anticancer Drugs 10:807–814
Pein F, Pinkerton R, Berthaud P, Pritchard-Jones K, Dick G, Vassal G (2007) Dose finding study of oral PSC 833 combined with weekly intravenous etoposide in children with relapsed or refractory solid tumours. Eur J Cancer 43:2074–2081
Hijiya N, Panetta JC, Zhou Y, Kyzer EP, Howard SC, Jeha S, Razzouk BI, Ribeiro RC, Rubnitz JE, Hudson MM, Sandlund JT, Pui CH, Relling MV (2006) Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 108:3997–4002
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167
Haddad S, Restieri C, Krishnan K (2001) Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Health A 64:453–464
Edginton AN, Theil FP, Schmitt W, Willmann S (2008) Whole body physiologically-based pharmacokinetic models: their use in clinical drug development. Expert Opin Drug Metab Toxicol 4:1143–1152
Teorell T (1937) The diffusion effect upon ionic distribution: II. Experiments on ionic accumulation. Gen Physiol 21:107–122
Bischoff KB, Dedrick RL, Zaharko DS (1970) Preliminary model for methotrexate pharmacokinetics. J Pharm Sci 59:149–154
Bischoff KB, Dedrick RL, Zaharko DS, Longstreth JA (1971) Methotrexate pharmacokinetics. J Pharm Sci 60:1128–1133
Benowitz N, Melmon KL, Rowland M (1974) Lidocaine disposition kinetics in monkey and man. I. Prediction by a perfusion model. Clin Pharmacol Ther 16:87–98
Edginton AN, Schmitt W, Voith B, Willmann S (2006) A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet 45:683–704
Edginton AN, Schmitt W, Willmann S (2006) Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet 45:1013–1034
Li J, Gwilt P (2003) The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol 51:395–402
Willmann S, Lippert S, Schmitt W (2005) From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol 1:159–168
Shah JC, Chen JR, Chow D (1989) Preformulation study of etoposide: identification of physicochemical characteristics responsible for the low and erratic oral bioavailability of etoposide. Pharm Res 6:408–412
Busse D, Würthwein G, Hinske C, Hempel G, Fromm MF, Eichelbaum M, Kroemer HK, Busch FW (2002) Pharmacokinetics of intravenous etoposide in patients with breast cancer: influence of dose escalation and cyclophosphamide and doxorubicin coadministration. Naunyn Schmiedebergs Arch Pharmacol 366:218–225
Rodgers T, Leahy D, Rowland M (2005) Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci 94:1259–1276
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257
Rodgers T, Rowland M (2007) Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res 24:918–933
Boos J, Krümpelmann S, Schulze-Westhoff P, Euting T, Berthold F, Jürgens H (1995) Steady-state levels and bone marrow toxicity of etoposide in children and infants: does etoposide require age-dependent dose calculation? J Clin Oncol 13:2954–2960
Kawashiro T et al (1998) A study on the metabolism of etoposide and possible interactions with antitumor or supporting agents by human liver microsomes. J Pharmacol Exp Ther 286:1294–1300
Watanabe Y, Nakajima M, Ohashi N, Kume T, Yokoi T (2003) Glucuronidation of etoposide in human liver microsomes is specifically catalyzed by UDP-glucuronosyltransferase 1A1. Drug Metab Dispos 31:589–595
Guo A, Marinaro W, Hu P, Sinko PJ (2002) Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT). Drug Metab Dispos 30:457–463
Hande KR (1992) Etoposide pharmacology. Semin Oncol 19(Suppl 13):3–9
Hayton WL (2000) Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci 2:E3
Clark PI, Slevin M (1987) The clinical pharmacology of etoposide and teniposide. Clin Pharmacokinet 12:223–252
Hande KR, Wolff SN, Greco FA, Hainsworth JD, Reed G, Johnson DH (1990) Etoposide kinetics in patients with obstructive jaundice. J Clin Oncol 8:1101–1107
Holthuis JJ (1988) Etoposide and teniposide. Bioanalysis, metabolism and clinical pharmacokinetics. Pharm Weekbl Sci 10:101–116
Pelekis M, Gephart LA, Lerman SE (2001) Physiological-model-based derivation of the adult and child pharmacokinetic intraspecies uncertainty factors for volatile organic compounds. Regul Toxicol Pharmacol 33:12–20
Price K, Haddad S, Krishnan S (2003) Physiological modeling of age-specific changes in the pharmacokinetics of organic chemicals in children. J Toxicol Environ Health A 66:417–433
Gentry PR, Covington TR, Clewell HJ 3rd (2003) Evaluation of the potential impact of pharmacokinetic differences on tissue dosimetry in offspring during pregnancy and lactation. Regul Toxicol Pharmacol 38:1–16
Bjorkman S (2005) Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol 59:691–704
Ginsberg G (2004) Physiologically based pharmacokinetic (PBPK) modeling of caffeine and theophylline in neonates and adults: implications for assessing children’s risks from environmental agents. J Toxicol Environ Health A 67:297–329
Lum BL, Kaubisch S, Yahanda AM, Adler KM, Jew L, Ehsan MN, Brophy NA, Halsey J, Gosland MP, Sikic BI (1992) Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol 10:1635–1642
Lacayo NJ, Lum BL, Becton DL, Weinstein H, Ravindranath Y, Chang MN, Bomgaars L, Lauer SJ, Sikic BI, Dahl GV (2002) Pharmacokinetic interactions of cyclosporine with etoposide and mitoxantrone in children with acute myeloid leukemia. Leukemia 16:920–927
Thomas HD, Porter DJ, Bartelink I, Nobbs JR, Cole M, Elliott S, Newell DR, Calvert AH, Highley M, Boddy AV (2002) Randomized cross-over clinical trial to study potential pharmacokinetic interactions between cisplatin or carboplatin and etoposide. Br J Clin Pharmacol 53:83–91
Rodman JH (1994) Altered etoposide pharmacokinetics and time to engraftment in pediatric patients undergoing autologous bone marrow transplantation. J Clin Oncol 12:2390–2397
Thiery-Vuillemin A, Dobi E, Nguyen T, Royer B, Montange D, Maurina T, Kalbacher E, Bazan F, Villanueva C, Demarchi M, Chaigneau L, Ivanaj A, Pivot X (2010) Duration: escalation study of oral etoposide with carboplatin in patients with varied solid tumors. Anticancer Drugs 21:958–962
Fenneteau F, Turgeon J, Couture L, Michaud V, Li J, Nekka F (2009) Assessing drug distribution in tissues expressing P-glycoprotein through physiologically based pharmacokinetic modeling: model structure and parameters determination. Theor Biol Med Model 6:2
Nishimura T, Kato Y, Amano N, Ono M, Kubo Y, Kimura Y, Fujita H, Tsuji A (2008) Species difference in intestinal absorption mechanism of etoposide and digoxin between cynomolgus monkey and rat. Pharm Res 25:2467–2476
Acknowledgments
The centre for clinical trials is funded by the German Federal Office for Research and Technology (BMBF, 01KN0705). This work was supported by Bayer Technology Services GmbH.
Conflict of interest
Stefan Willmann and Jörg Lippert are employees of Bayer Technology Services. Georg Hempel and Gisela Kersting received funding from Bayer Technology Services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kersting, G., Willmann, S., Würthwein, G. et al. Physiologically based pharmacokinetic modelling of high- and low-dose etoposide: from adults to children. Cancer Chemother Pharmacol 69, 397–405 (2012). https://doi.org/10.1007/s00280-011-1706-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1706-9