Abstract
Purpose
Bosutinib, a dual Src/Abl kinase inhibitor in development for treatment of chronic myeloid leukemia, is primarily metabolized by the CYP3A4 hepatic enzyme. This study evaluated the pharmacokinetics and safety of bosutinib in patients with chronic hepatic impairment and matched healthy subjects.
Methods
Hepatically impaired patients were aged 18–65 years and of Child-Pugh classes A, B, or C; healthy subjects were matched by age, sex, body mass index, and smoking habits. A single oral dose of bosutinib 200 mg was administered on day 1 within 5 min after completion of breakfast.
Results
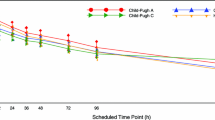
Compared with healthy subjects (n = 9), maximal plasma concentration (C max) and area under the curve increased 2.42-fold and 2.25-fold in Child-Pugh A (n = 6), 1.99-fold and 2.0-fold in Child-Pugh B (n = 6), and 1.52-fold and 1.91-fold in Child-Pugh C patients (n = 6). Time to C max decreased from 4 h in healthy subjects to 2.5, 2.0, and 1.5 h in Child-Pugh A, B, and C patients, respectively; the elimination half-life increased from 55 h in healthy subjects to 86, 113, and 111 h in Child-Pugh A, B, and C patients. Bosutinib oral clearance was lower in hepatically impaired patients compared with healthy subjects. Frequently reported adverse events included prolonged QTc interval (37.0 %, n = 10), nausea (11.1 %, n = 3), and vomiting (7.4 %, n = 2).
Conclusions
A single oral dose of bosutinib 200 mg showed acceptable tolerability in healthy subjects and in patients with mild, moderate, or severe chronic hepatic impairment.

Similar content being viewed by others
References
Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602:114–130
Pendergast AM (2002) The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res 85:51–100
Saad F, Lipton A (2010) SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev 36:177–184
Shakespeare WC, Metcalf CA III, Wang Y, Sundaramoorthi R, Keenan T, Weigele M, Bohacek RS, Dalgarno DC, Sawyer TK (2003) Novel bone-targeted Src tyrosine kinase inhibitor drug discovery. Curr Opin Drug Discov Devel 6:729–741
Hantschel O, Superti-Furga G (2004) Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 5:33–44
Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F (2001) Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem 44:3965–3977
Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F (2003) SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 63:375–381
Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA (2007) A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res 67:1580–1588
Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D (2011) Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol 51:1721–1727
Abbas-Borhan R, Chaudhary I, Hug BA, Leister C, Burns J, Vashishtha S, Erve JCL, Sonnichsen D (2010) Mass balance, metabolic disposition, metabolite characterization, and pharmacokinetics of oral 14C-labeled bosutinib in healthy subjects. Presented at: the 9th Triennial Meeting of the International Society for the Study of Xenobiotics; September 4–8, 2010; Istanbul, Turkey. Abstract P350
Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, Sonnichsen D (2012) A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother Pharmacol 69:221–227
Daud AI, Krishnamurthi SS, Saleh MN, Gitlitz BJ, Borad MJ, Gold PJ, Chiorean EG, Springett GM, Abbas R, Agarwal S, Bardy-Bouxin N, Hsyu PH, Leip E, Turnbull K, Zacharchuk C, Messersmith WA (2012) Phase I study of bosutinib, a Src/Abl tyrosine kinase inhibitor, administered to patients with advanced solid tumors. Clin Cancer Res 18:1092–1100
Cortes JE, Kim D-W, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicus L, Malhotra H, Powell C, Gogat K, Countouriotis AM, Gambacorti-Passerini C (2012) Bosutinib versus imatinib in newly diagnosed chronic phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol (Epub ahead of print)
Cortes JE, Kantarjian HM, Brummendorf TH, Kim D-W, Turkina AG, Shen Z-X, Pasquini R, Khoury HJ, Arkin S, Volkert A, Besson N, Abbas R, Wang J, Leip E, Gambacori-Passerini C (2011) Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 118:4567–4576
Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini CB, Baccarani M, Kim DW, Zaritskey A, Countouriotis A, Besson N, Leip E, Kelly V, Brummendorf TH (2012) Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood 119:3403–3412
Food and Drug Administration (2011) Drug development and drug interactions: table of substrates, inhibitors and inducers. http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm#inVivo. Accessed 3 Feb 2012
Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Moller S (2002) Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol 36:513–520
Kosar F, Ates F, Sahin I, Karincaoglu M, Yildirim B (2007) QT interval analysis in patients with chronic liver disease: a prospective study. Angiology 58:218–224
Mohamed R, Forsey PR, Davies MK, Neuberger JM (1996) Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology 23:1128–1134
Zambruni A, Di Micoli A, Lubisco A, Domenicali M, Trevisani F, Bernardi M (2007) QT interval correction in patients with cirrhosis. J Cardiovasc Electrophysiol 18:77–82
Finucci G, Lunardi F, Sacerdoti D, Volpin R, Bortoluzzi A, Bombonato G, Angeli P, Gatta A (1998) Q-T interval prolongation in liver cirrhosis. Reversibility after orthotopic liver transplantation. Jpn Heart J 39:321–329
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2003) Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072123.pdf. Accessed 3 Feb 2012
van Erp NP, Gelderblom H, Guchelaar HJ (2009) Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 35:692–706
George J, Murray M, Byth K, Farrell GC (1995) Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology 21:120–128
Haouala A, Widmer N, Duchosal MA, Montemurro M, Buclin T, Decosterd LA (2011) Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 117:e75–e87
Campone M, Bondarenko I, Brincat S, Hotko Y, Munster PN, Chmielowska E, Fumoleau P, Ward R, Bardy-Bouxin N, Leip E, Turnbull K, Zacharchuk C, Epstein RJ (2012) Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann Oncol 23:610–617
Yap YG, Camm AJ (2003) Drug induced QT prolongation and torsades de pointes. Heart 89:1363–1372
Roden DM (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350:1013–1022
Ponte ML, Keller GA, Di Girolamo G (2010) Mechanisms of drug induced QT interval prolongation. Curr Drug Saf 5:44–53
Abbas R, Hug B, Leister C, Burns J, Sonnichsen D (2010) A single dose, crossover, placebo- and moxifloxacin-controlled study to assess the effects of bosutinib (SKI-606) on cardiac repolarization in healthy adult subjects. Poster presented at the 15th Congress of the European Hematology Association; June 10–13, 2010; Barcelona, Spain. Abstract 0846
Acknowledgments
We thank the study participants as well as the research personnel at the study site. This study was sponsored by Wyeth Research, which was acquired by Pfizer Inc in October 2009. Richat Abbas is an employee of Pfizer, and Stephan Chalon, Cathie Leister, Myriam El Gaaloul, and Daryl Sonnichsen are former employees of Pfizer; through employment of the authors, the sponsor was involved in all aspects of study design and conduct as well as manuscript preparation. Stephan Chalon, Cathie Leister, and Daryl Sonnichsen additionally own/owned stock in Pfizer. Medical writing support was provided by Kimberly Brooks, PhD, of SciFluent, and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abbas, R., Chalon, S., Leister, C. et al. Evaluation of the pharmacokinetics and safety of bosutinib in patients with chronic hepatic impairment and matched healthy subjects. Cancer Chemother Pharmacol 71, 123–132 (2013). https://doi.org/10.1007/s00280-012-1987-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1987-7