Abstract
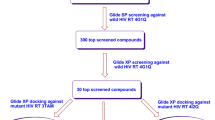
Molecular docking is a reliable method with which to identify the binding conformations of substrates, inducers and inhibitors of cytochrome P450 (CYP) enzymes. We used the docking method to explore possible binding modes of an entry inhibitor (maraviroc) and non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz and etravirine) to cytochrome P450 3A4 (CYP3A4). In addition, docking results were compared with the binding conformations of HIV protease drugs to infer the binding site residues and potential drug–drug interaction profiles for combination therapy in the treatment of AIDS. We observed that efavirenz and etravirine induce metabolism of co-administered drugs by binding to a unique position in the active site of CYP3A4. Dosage adjustment is required for delavirdine and maraviroc when combined with HIV protease drugs. The present results are in good agreement with experimental data from drug interaction profiles. The information provided in this paper will be helpful in furthering our understanding the functions of CYP3A4, and could aid in the design of new drugs that would be metabolized easily without having any drug–drug interaction profile.

The docked poses of etravirine (ETR) inside the active site of cytochrome P450 3A4 (CYP3A4). The binding site residues are colored by atom type: gray carbon, red oxygen, blue nitrogen, cyan hydrogen, yellow sulfur. The sticks of the ligands and heme are colored in green.



Similar content being viewed by others
References
Sterne JA, Hernan MA, Ledergerber B, Tilling K, Weber R, Sendi P, Rickenbach M, Robins JM, Egger M (2005) Long-term effectiveness of potent antiretroviral therapy in preventing aids and death: a prospective cohort study. Lancet 366:378–384. doi:10.1016/S0140-6736(05)67022-5
Gange SJ, Barron Y, Greenblatt RM, Anastos K, Minkoff H, Young M, Kovacs A, Cohen M, Meyer WA 3rd, Munoz A (2002) Effectiveness of highly active antiretroviral therapy among hiv-1 infected women. J Epidemiol Commun Health 56:153–159. doi:10.1136/jech.56.2.153
Sheweita SA (2000) Drug-metabolizing enzymes: mechanisms and functions. Curr Drug Metab 1:107–132. doi:10.2174/1389200003339117
Brown KC, Paul S, Kashuba ADM (2009) Drug interactions with new and investigational antiretrovirals. Clin Pharmacokinet 48:211–241. doi:10.2165/00003088-200948040-00001
Tanaka E (1998) Clinically important pharmacokinetic drug-drug interactions: role of cytochrome p450 enzymes. J Clin Pharm Ther 23:403–416. doi:10.1046/j.1365-2710.1998.00086x
Kjellander B, Masimirembwa CM, Zamora I (2007) Exploration of enzyme-ligand interactions in cyp2d6 and 3a4 homology models and crystal structures using a novel computational approach. J Chem Inf Model 47:1234–1247. doi:10.1021/ci600561v
Oda A, Yamaotsu N, Hirono S (2004) Studies of binding modes of (s)-mephenytoin to wild types and mutants of cytochrome P450 2 C19 and 2 C9 using homology modeling and computational docking. Pharm Res 21:2270–2278. doi:10.1007/s11095-004-7680-8
Ito Y, Kondo H, Goldfarb PS, Lewis DF (2008) Analysis of cyp2d6 substrate interactions by computational methods. J Mol Graph Model 26:947–956. doi:10.1016/j.jmgm.2007.07.004
Yao Y, Han WW, Zhou YH, Li ZS, Li Q, Chen XY, Zhong DF (2009) The metabolism of cyp2c9 and cyp2c19 for gliclazide by homology modeling and docking study. Eur J Med Chem 44:854–861. doi:10.1016/j.ejmech.2008.04.015
Jayakanthan M, Chandrasekar S, Muthukumaran J, Mathur PP (2010) Analysis of cyp3a4-hiv-1 protease drugs interactions by computational methods for highly active antiretroviral therapy in hiv/aids. J Mol Graph Model 28:455–463. doi:10.1016/j.jmgm.2009.10.005
Berman H, Henrick K, Nakamura H, Markley JL (2007) The worldwide protein data bank (wwpdb): ensuring a single, uniform archive of pdb data. Nucleic Acids Res 35:D301–303. doi:10.1093/nar/gkl971
Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, Johnson EF (2004) The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-Å resolution. J Biol Chem 279:38091–38094. doi:10.1074/jbc.C400293200
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662. doi:10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Scott EE, Halpert JR (2005) Structures of cytochrome p450 3a4. Trends Biochem Sci 30:5–7. doi:10.1016/j.tibs.2004.11.004
Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, Tickle IJ, Jhoti H (2004) Crystal structures of human cytochrome p450 3a4 bound to metyrapone and progesterone. Science 305:683–686. doi:10.1126/science.1099736
Kapelyukh Y, Paine MJ, Marechal JD, Sutcliffe MJI, Wolf CR, Roberts GCK (2008) Multiple substrate binding by cytochrome p450 3a4: estimation of the number of bound substrate molecules. Drug Metab Dispos 36:2136–2144. doi:10.1124/dmd.108.021733
Korzekwa KR, Krishnamachary N, Shou M, Ogai A, Parise RA, Rettie AE, Gonzalez FJ, Tracy TS (1998) Evaluation of atypical cytochrome p450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome p450 active sites. Biochemistry 37:4137–4147. doi:10.1021/bi9715627
Shou M, Grogan J, Mancewicz JA, Krausz KW, Gonzalez FJ, Gelboin HV, Korzekwa KR (1994) Activation of CYP3A4: evidence for the simultaneous binding of two substrates in a cytochrome p450 active site. Biochemistry 33:6450–6455. doi:10.1021/bi00187a009
Gibbs MA, Hosea NA (2003) Factors affecting the clinical development of cytochrome p450 3a substrates. Clin Pharmacokinet 42:969–984
Fowler SM, Taylor JM, Friedberg T, Wolf CR, Riley RJ (2002) CYP3A4 active site volume modification by mutagenesis of leucine 211. Drug Metab Dispos 30:452–456. doi:10.1124/dmd.30.4.452
Fowler SM, Riley RJ, Pritchard MP, Sutcliffe MJ, Friedberg T, Wolf CR (2000) Amino acid 305 determines catalytic center accessibility in CYP3A4. Biochemistry 39:4406–4414. doi:10.1021/bi992372u
Roussel F, Khan KK, Halpert JR (2000) The importance of SRS-1 residues in catalytic specificity of human cytochrome P450 3A4. Arch Biochem Biophys 374:269–278. doi:10.1006/abbi.1999.1599
Tran JQ, Gerber JG, Kerr BM (2001) Delavirdine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet 40:207–226. doi:10.1006/abbi.1999.1599
Harris M, Alexander C, O'Shaughnessy M, Montaner JSG (2002) Delavirdine increases drug exposure of ritonavir-boosted protease inhibitors. AIDS 16:798–799
Scott LJ, Perry CM (2000) Delavirdine: a review of its use in HIV infection. Drugs 60:1411–1444
Gilden D (1996) Delavirdine/protease inhibitor interactions. GMHC Treat Issues 10:11–12
Best BM, Goicoechea M (2008) Efavirenz - still first-line king? Expert Opin Drug Metab Toxicol 4:965–972. doi:10.1517/17425255.4.7.965
Dailly E, Allavena C, Raffi F, Jolliet P (2005) Pharmacokinetic evidence for the induction of lopinavir metabolism by efavirenz. Br J Clin Pharmacol 60:32–34. doi:10.1111/j.1365-2125.2005.02369.x
Pfister M, Labbe L, Lu JF, Hammer SM, Mellors J, Bennett KK, Rosenkranz S, Sheiner LB (2002) Effect of coadministration of nelfinavir, indinavir, and saquinavir on the pharmacokinetics of amprenavir. Clin Pharmacol Ther 72:133–141. doi:10.1067/mcp.2002.126183
Pham PA, Hendrix CW, Barditch-Crovo P, Parsons T, Khan W, Parish M, Radebaugh C, Carson KA, Pakes GE, Qaqish R, Flexner C (2007) Amprenavir and lopinavir pharmacokinetics following coadministration of amprenavir or fosamprenavir with lopinavir/ritonavir, with or without efavirenz. Antivir Ther 12:963–969
Martinez E, Nelson M (2010) Simplification of antiretroviral therapy with etravirine. AIDS Rev 12:52–59
Tseng A, MacArthur RD (2010) Profile of etravirine for the treatment of hiv infection. Ther Clin Risk Manag 6:49–58. doi:10.2147/TCRM.S3128
Perez VE, Sanchez-Parra C, Villar SS (2009) Etravirine drug interactions. Enferm Infecc Microbiol Clín 27(Suppl 2):27–31. doi:10.1016/S0213-005X(09)73216-1
Peytavin G (2008) clinical pharmacokinetic of maraviroc. Méd Mal Infect 38(Suppl 1):S12–S16. doi:10.1016/S0399-077X(08)70539-0
Abel S, Jenkins TM, Whitlock LA, Ridgway CE, Muirhead GJ (2008) Effects of cyp3a4 inducers with and without cyp3a4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol 65(Suppl 1):38–46. doi:10.1111/j.1365-2125.2008.03134.x
Abel S, Russell D, Taylor-Worth RJ, Ridgway CE, Muirhead GJ (2008) Effects of cyp3a4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol 65(Suppl 1):27–37. doi:10.1111/j.1365-2125.2008.03133.x
Acknowledgments
P.P. Mathur acknowledges receipt of financial support from the Department of Biotechnology (DBT), and the Department of Information Technology (DIT), Government of India, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mannu, J., Jenardhanan, P. & Mathur, P.P. A computational study of CYP3A4 mediated drug interaction profiles for anti-HIV drugs. J Mol Model 17, 1847–1854 (2011). https://doi.org/10.1007/s00894-010-0890-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0890-6