Abstract
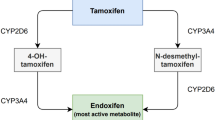
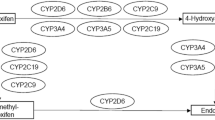
Endocrine therapy for hormone-sensitive breast cancer is a well-established treatment option, both in adjuvant and palliative settings. For patients undergoing chronic hemodialysis, only scant pharmacokinetic data have been published for tamoxifen, and no data have been published for anastrozole. We therefore measured plasma levels of tamoxifen, its major metabolite, N-desmethyl tamoxifen, and anastrozole in a breast cancer patient undergoing chronic hemodialysis. Clinical tolerability was good. The blood levels for tamoxifen, N-desmethyl tamoxifen and anastrozole were within the expected therapeutic ranges. From this study, we can conclude that endocrine therapy for breast cancer with tamoxifen or anastrozole seems feasible and safe for patients undergoing chronic hemodialysis.
Similar content being viewed by others
References
Morello KC, Wurz GT, DeGregorio MW (2003) Pharmacokinetics of selective estrogen receptor modulators. Clin Pharmacokinet 42(4):362–372
Bonneterre J, Buzdar A, Nabholtz JM et al (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Results of two randomised trials designed for combined analysis. Cancer 92(9):2247–2258
Chem Bank (2002) Global database for potentially hazardous chemicals http://www.chembank.med.harvard.edu. Cited 22 Mar 2006
Crewe HK, Noteley LM, Wunsch RM et al (2002) Metabolism of tamoxifen by recombinant human cytochrome p450 enzymes: formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30(8):869–874
Sheth HR, Lord G, Tkaczuk K et al (2003) Aging may be associated with concentrations of tamoxifen and its metabolites in breast cancer patients. J Womens Health (Larchmt) 2(8):799–808
Guerrieri-Gonzaga A, Baglietto L, Johansson H, Bonanni B, Robertson C, Sandri MT et al (2001) Correlation between tamoxifen elimination and biomarker recovery in a primary prevention trial. Cancer Epidemiol Biomarkers Prev 10:967–970
Sutherland CM, Sternson LA, Muchmore JH et al (1984) Effect of impaired renal function on tamoxifen. J Surg Oncol 27:222–223
Allaria PM, Giangrande A, Gandini E et al (1999) Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol 12:395–397
Buzdar AU, Robertson JFR, Eiermann W et al (2002) An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole and exemestane. Cancer 95(9):2006–2016
Lonning P, Pfister C, Martoni A et al (2003) Pharmacokinetics of third-generation aromatase inhibitors. Sem Oncol 30(4, Supp 14):23–32
The ATAC Trialist Group (2001) Pharmacokinetics of anastrazole and tamoxifen alone, and in combination, during adjuvant endocrine therapy for early breast cancer in postmenopausal women: a sub-protocol of the “Arimidex and Tamoxifen Alone or in Combination” (ATAC) trial. Br J Cancer 85(3):317–324
Pastan S, Bailey J (1998) Dialysis therapy. NEJM 338(20):1428–1437
Remmele W, Stegner HE (1987) Vorschlag zur einheitlichen Definition eines Immunreaktiven Score (IRS) für den immunhistochemischen Östrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe 8:138–140
Decensi A, Gandini S, Guerrieri-Gonzaga A (1999) Effect of blood tamoxifen concentrations on surrogate biomarkers in a trial of dose reduction in healthy women. J Clin Oncol 17(9):2633–2638
Fagugli RM, De Smet R, Buoncristiani U (2002) Behavior of non-protein-bound and protein-bound uremic solutes during daily hemodialysis. Am J Kidney Dis 40(2):339–347
Acknowledgment
This study was supported by a grant form Astra Zeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Langenegger, T., Wahl, P., Schiesser, D. et al. Plasma Levels of Tamoxifen, N-Desmethyl Tamoxifen and Anastrozole in a Patient with Metastatic Breast Cancer and Chronic Hemodialysis. Breast Cancer Res Treat 100, 177–181 (2006). https://doi.org/10.1007/s10549-006-9243-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9243-7