Abstract
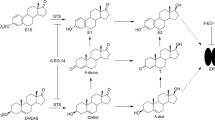
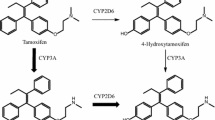
The mechanism of tamoxifen action in the treatment of breast cancer is believed to be via active metabolites that act as potent estrogen receptor antagonists. Attempts to identify relationships between active metabolite concentrations and clinical outcomes have produced mixed results. Since anti-estrogenic effects may be brought about not only by estrogen antagonism, but also by reduced estrogen synthesis, we tested the ability of tamoxifen and its principal metabolites to inhibit aromatase in vitro. The activity of human aromatase in both recombinant and placental microsomal preparations was measured using the rate of generation of a fluorescent metabolite in the presence and absence of multiple concentrations of tamoxifen, endoxifen, N-desmethyl-tamoxifen, and Z-4-hydroxy-tamoxifen. Aromatase inhibition was further characterized by measuring the inhibition of testosterone metabolism to estradiol. The biochemical mechanisms of inhibition were documented and their inhibitory potency was compared. Using recombinant human aromatase, endoxifen, and N-desmethyl-tamoxifen were able to inhibit aromatase activity with K i values of 4.0 and 15.9 μM, respectively. Detailed characterization of inhibition by endoxifen and N-desmethyl-tamoxifen indicated non-competitive kinetics for both inhibitors. Similarly, endoxifen-inhibited testosterone metabolism via a non-competitive mechanism. No appreciable inhibition by tamoxifen or Z-4-hydroxy-tamoxifen was observed at similar concentrations. The relative inhibitory potency was: endoxifen > N-desmethyl-tamoxifen >>> Z-4-hydroxy-tamoxifen > tamoxifen. Similar data were obtained in human placental microsomes. Endoxifen and N-desmethyl-tamoxifen were found to be potent inhibitors of aromatase. Inhibition by these tamoxifen metabolites may contribute to the variability in clinical effects of tamoxifen in patients with breast cancer. Relationships between tamoxifen metabolite concentrations and clinical outcomes may be complex, and the biologic mechanisms that underlie these relationships may include aromatase inhibition.





Similar content being viewed by others
Abbreviations
- Endoxifen:
-
4-Hydroxy-N-desmethyl-tamoxifen
- 4HT:
-
Z-4-hydroxy-tamoxifen
- NDMT:
-
N-desmethyl-tamoxifen
- CYP:
-
Cytochrome P450
- CYP19:
-
Aromatase
- MFC:
-
7-Methoxy-4-trifluoromethylcoumarin
- HFC:
-
7-Hydroxytrifluoromethylcoumarin
- HPLC:
-
High performance liquid chromatography
- UV:
-
Ultraviolet
- IC50 :
-
The half maximal inhibitory concentration
- K m :
-
The Michaelis constant
- V max :
-
The maximum reaction rate
- K i :
-
The equilibrium dissociation constant of the inhibitor
- K Sapp :
-
The apparent Michaelis constant
- V maxi :
-
The apparent maximum reaction rate in the presence of the inhibitor
References
Furr BJ, Jordan VC (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 25(2):127–205. doi:0163-7258(84)90043-3
Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, Serrano D, Pelosi G, Decensi A, Lien EA (2004) Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res 10(7):2336–2343
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97(1):30–39. doi:10.1093/jnci/dji005
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95(23):1758–1764
Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23(36):9312–9318. doi:10.1200/JCO.2005.03.3266
Wegman P, Elingarami S, Carstensen J, Stal O, Nordenskjold B, Wingren S (2007) Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res 9(1):R7. doi:10.1186/bcr1640
Higgins MJ, Stearns V (2010) CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr Oncol Rep 12(1):7–15. doi:10.1007/s11912-009-0076-5
Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C, MacLeod SL, Kadlubar FF, Ambrosone CB (2005) Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 91(3):249–258. doi:10.1007/s10549-004-7751-x
Higgins MJ, Rae JM, Flockhart DA, Hayes DF, Stearns V (2009) Pharmacogenetics of tamoxifen: Who should undergo CYP2D6 genetic testing? J Natl Compr Canc Netw 7(2):203–213
Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, Pierce JP (2008) Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 108(3):421–426. doi:10.1007/s10549-007-9612-x
Henry NL, Rae JM, Li L, Azzouz F, Skaar TC, Desta Z, Sikora MJ, Philips S, Nguyen AT, Storniolo AM, Hayes DF, Flockhart DA, Stearns V (2009) Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat 117(3):571–575. doi:10.1007/s10549-009-0309-1
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N (2005) Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 97(22):1652–1662. doi:10.1093/jnci/dji372
Thompson AM, Johnson A, Quinlan P, Hillman G, Fontecha M, Bray SE, Purdie CA, Jordan LB, Ferraldeschi R, Latif A, Hadfield KD, Clarke RB, Ashcroft L, Evans DG, Howell A, Nikoloff M, Lawrence J, Newman WG (2010) Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. doi:10.1007/s10549-010-1139-x
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ (2010) American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28(23):3784–3796. doi:10.1200/JCO.2009.26.3756
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62. doi:10.1016/S0140-6736(04)17666-6
Zivian MT, Salgado B (2008) Side effects revisited: women’s experiences with aromatase inhibitors. A report from Breast Cancer Action. San Francisco
Lonning PE, Johannessen DC, Lien EA, Ekse D, Fotsis T, Adlercreutz H (1995) Influence of tamoxifen on sex hormones, gonadotrophins and sex hormone binding globulin in postmenopausal breast cancer patients. J Steroid Biochem Mol Biol 52(5):491–496. doi:0960-0760(94)00189-S
Lu WJ, Bies R, Kamden LK, Desta Z, Flockhart DA (2010) Methadone: a substrate and mechanism-based inhibitor of CYP19 (aromatase). Drug Metab Dispos 38(8):1308–1313. doi:10.1124/dmd.110.032474
Stresser DM (2004) High-throughput screening of human cytochrome P450 inhibitors using fluorometric substrates. In: Caldwell ZYGW (ed) Optimization in drug discovery: in vitro methods. Methods in pharmacology and toxicology. Humana Press, Totowa, pp 215–230. doi:10.1385/1-59259-800-5:215
Segel IH (1993) Enzyme kinetics. Wiley, New York
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80(1):61–74. doi:10.1016/j.clpt.2006.03.013
Lien EA, Wester K, Lonning PE, Solheim E, Ueland PM (1991) Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer 63(4):641–645
Lien EA, Solheim E, Ueland PM (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 51(18):4837–4844
Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM (1989) Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res 49(8):2175–2183
Jordan VC (1982) Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat 2(2):123–138
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85(2):151–159. doi:10.1023/B:BREA.0000025406.31193.e8
Ahmad A, Shahabuddin S, Sheikh S, Kale P, Krishnappa M, Rane RC, Ahmad I (2010) Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther 88(6):814–817. doi:10.1038/clpt.2010.196
Zharikova OL, Deshmukh SV, Nanovskaya TN, Hankins GD, Ahmed MS (2006) The effect of methadone and buprenorphine on human placental aromatase. Biochem Pharmacol 71(8):1255–1264. doi:10.1016/j.bcp.2005.12.035
Ghosh D, Griswold J, Erman M, Pangborn W (2010) X-ray structure of human aromatase reveals an androgen-specific active site. J Steroid Biochem Mol Biol 118(4–5):197–202. doi:10.1016/j.jsbmb.2009.09.012
Payne EJ, Ingley E, Dick IM, Wilson SG, Bond CS, Prince RL (2009) In vitro kinetic properties of the Thr201Met variant of human aromatase gene CYP19A1: functional responses to substrate and product inhibition and enzyme inhibitors. J Clin Endocrinol Metab 94(8):2998–3002. doi:10.1210/jc.2008-2309
Love RR, Nguyen BD, Nguyen CB, Nguyen VD, Havighurst TC (1999) Symptoms associated with oophorectomy and tamoxifen treatment for breast cancer in premenopausal Vietnamese women. Breast Cancer Res Treat 58(3):281–286
Acknowledgments
The authors are grateful to Evan Ogburn MSc for providing technical assistance. This study was supported in part by the National Institutes of Health National Center for Research Resources [Grant K24RR020815] to DF, by the National Institute for General Medical Sciences [Grants T32GM008425, U01GM061373] to DF, and by the Department of Defense Breast Cancer Research Program Predoctoral Fellowship [W81XWH-11-1-0016] to WL.
Conflict of interest
WJL and DAF are authors of a patent submitted to the U.S. patent office that describes new uses of the chemical structures described herein entitled “Materials for inhibiting aromatase and method of using the same to diagnose, treat and monitor breast cancer”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, W.J., Desta, Z. & Flockhart, D.A. Tamoxifen metabolites as active inhibitors of aromatase in the treatment of breast cancer. Breast Cancer Res Treat 131, 473–481 (2012). https://doi.org/10.1007/s10549-011-1428-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1428-z