Abstract
(1) We evaluated the involvement of brain mitochondrial and microsomal cytochrome P-450 in the metabolization of known porphyrinogenic agents, with the aim of improving the knowledge on the mechanism leading to porphyric neuropathy. We also compared the response in brain, liver and kidney. To this end, we determined mitochondrial and microsomal cytochrome P-450 levels and the activity of NADPH cytochrome P-450 reductase. (2) Animals were treated with known porphyrinogenic drugs such as volatile anaesthetics, allylisopropylacetamide, veronal, griseofulvin and ethanol or were starved during 24 h. Cytochrome P-450 levels and NADPH cytochrome P-450 reductase activity were measured in mitochondrial and microsomal fractions from the different tissues. (3) Some of the porphyrinogenic agents studied altered mitochondrial cytochrome P-450 brain but not microsomal cytochrome P-450. Oral griseofulvin induced an increase in mitochondrial cytochrome P-450 levels, while chronic Isoflurane produced a reduction on its levels, without alterations on microsomal cytochrome P-450. Allylisopropylacetamide diminished both mitochondrial and microsomal cytochrome P-450 brain levels; a similar pattern was detected in liver. Mitochondria cytochorme P-450 liver levels were only diminished after chronic Isoflurane administration. In kidney only mitochondrial cytochrome P-450 levels were modified by veronal; while in microsomes, only acute anaesthesia with Enflurane diminished cytochrome P-450 content. (4) Taking into account that δ-aminolevulinic acid would be responsible for porphyric neuropathy, we investigated the effect of acute and chronic δ-aminolevulinic acid administration. Acute δ-aminolevulinic acid administration reduced brain and liver cytochrome P-450 levels in both fractions; chronic δ-aminolevulinic acid administration diminished only liver mitochondrial cytochrome P-450. (5) Brain NADPH cytochrome P-450 reductase activity in animals receiving allylisopropylacetamide, dietary griseofulvin and δ-aminolevulinic acid showed a similar profile as that for total cytochrome P-450 levels. The same response was observed for the hepatic enzyme. (6) Results here reported revealed differential tissue responses against the xenobiotics assayed and give evidence on the participation of extrahepatic tissues in porphyrinogenic drug metabolization. These studies have demonstrated the presence of the integral Phase I drug metabolizing system in the brain, thus, total cytochrome P-450 and associated monooxygenases in brain microsomes and mitochondria would be taken into account when considering the xenobiotic metabolizing capability of this organ.






Similar content being viewed by others
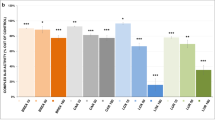
References
Anandatheerthavarada HK, Shankar SK, Ravindranath V (1990) Rat brain cytochromes P-450: catalytic, immunochemical properties and inducibility of multiple forms. Brain Res 526:339–343
Anandatheerthavarada HK, Shankar SK, Bhamre S, Boyd MR, Song BJ, Ravindranath V (1993) Induction of brain cytochrome P-450IIE1 by chronic ethanol treatment. Brain Res 601:279–285
Batlle AM (1997) Porfirias y Porfirinas. Aspectos clínicos, bioquímicos y biología molecular. Acta Bioquím. Clin. Latinoam. Suppl. N° 3
Bergh AF, Strobel HW (1992) Reconstitution of the brain mixed function oxidase system: purification of NADPH-cytochrome P450 reductase and partial purification of cytochrome P450 from whole brain. J Neurochem 59:575–581
Bhagwat SV, Boyd MR, Ravindranath V (2000) Multiple forms of cytochrome P450 and associated monooxygenase activities in human brain mitochondria. Biochem Pharmacol 59:573–582
Buzaleh AM, Vazquez ES, Nuñez G, Batlle AM (1997) Effect of chronic anesthesia on the drug-metabolizing enzime system and heme pathway regulation. Gen Pharmac 28:577–582
De Matteis F, Marks GS (1996) Cytochrome P450 and its interactions with the heme biosynthetic pathway. Can J Physiol Pharmacol 74:1–8
Ennis SR, Novotny A, Xiang J, Shakui P, Masada T, Stummer W, Smith DE, Keep RF (2003) Transport of 5-aminolevulinic acid between blood and brain. Brain Res 959:226–234
Garcia SC, Moretti MB, Garay MV, Batlle A (1998) Delta-aminolevulinic acid transport through blood-brain barrier. Gen Pharmacol 31:579–582
Gervasini G, Carrillo JA, Benitez J (2004) Potential role of cerebral cytochrome P450 in clinical pharmacokinetics. Clin Pharmacokinet 43:693–706
Ghersi-Egea JF, Perrin R, Leininger-Muller B, Grassiot MC, Jeandel C, Floquet J, Cuny G, Siest G, Minn A (1993) Subcellular localization of cytochrome P450, and activities of several enzymes responsible for drug metabolism in the human brain. Biochem Pharmacol 45:647–658
Guengerich FP (2003) Cytochromes P450, drugs, and diseases. Mol Interv 3:194–204
Honkakoski P, Kojo A, Raunio H, Pasanen M, Juvonen R, Lang MA (1988) Hepatic mitochondrial coumarin 7-hydroxylase comparison with the microsomal enzyme Arch Biochem Biophys 267:558–567
Johannesen KA, DePierre JW (1978) Measurement of cytochrome P-450 in the presence of large amounts of contaminating hemoglobin and methemoglobin. Anal Biochem 86:725–732
Lieber ES (1997) Cytochrome P-4502E1; its physiological and pathological role. Physiol Rev 77:517–544
Lin JH, Lu A (1998) Inhibition and Induction of Cytochrome P450 and the clinical implications. Clin Pharmacokinet 35:361–390
Loeper J, Descatoire V, Maurice M, Beaune P, Feldmann G, Larrey D, Pessayre D (1990) Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. J Hepatology 11:850–858
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Masters BSS, Williams Jr, CH, Kamin H (1967) The preparation and properties of microsomal TPNH-Cytochrome c Reductase from pig liver. In Estabrook RW, Pullman ME (eds), Methods in Enzymology, Elsevier Inc, Vol. 10, pp 565–573
Miksys SL, Rao Y, Sellers EM, Kwan M, Mendis D, Tyndale RF (2000) Regional and cellular distribution of CYP2D family members in rat brain. Xenobiotica 30:547–564
Miksys SL, Tyndale RE (2002) Drug-metabolizing cytochrome P450s in the brain. Rev Psychiatr Neurosci 27:406–415
Montoliu C, Valles S, Renau-Piqueras J, Guerri C (1994) Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem 63:1855–1862
Nabeshima T, Fontenot J, Ho IK (1981) Effects of chronic administration of pentobarbital or morphine on the brain microsomal cytochrome P-450 system. Biochem Pharmacol 30:1142–1145
Neve EP, Eliasson E, Pronzato MA, Albano E, Marinari U, Ingelman-Sundberg M (1996) Enzyme specific transport of rat liver cytochrome P450 in the golgi apparatus. Arch Biochem Biophys 333:459–465
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. J Biol Chem 239:2370–2378
Phillips AH, Langdon RG (1962) Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem 237:2652–2660
Ravindranath V, Anandatheerthavarada HK, Shankar SK (1989) Xenobiotic metabolism in human brain-presence of cytochrome P-450 and associated mono-oxygenases. Brain Res 496:331–335
Ravindranath V, Bhamre S, Bhagwat SV, Anandatheerthavarada HK, Shankar SK, Tirumalai PS (1995) Xenobiotic metabolism in brain. Toxicol Lett 82/83:633–638
Rodriguez JA, Buzaleh AM, Fossati M, Azcurra J, Batlle AM (2002) The effects of some porphyrinogenic drugs on the brain cholinergic system. Cell Mol Biol 48:103–110
Rodriguez JA, Martinez Mdel C, Gerez E, Batlle A, Buzaleh AM (2005) Heme oxygenase, aminolevulinate acid synthetase and the antioxidant system in the brain of mice treated with porphyrinogenic drugs. Cell Mol Biol 51:1–8
Rosenbrock H, Hagemeyer CE, Singec I, Knoth R, Volk B (1999) Testosterone metabolism in rat brain is differentially enhanced by phenytoin-inducible cytochrome P450 isoforms. J Neuroendocrinol 11:597–604
Thunell S (2000) Porphyrins, porphyrin metabolism and porphyrias. I. Update. Scand J Clin Lab Invest 60:509–540
Walther B, Ghersi-Egea JF, Minn A, Siest G (1986) Subcellular distribution of Cytochrome P-450 in the brain. Brain Res 375:338–344
Acknowledgements
A. M. Buzaleh and A. Batlle hold the post of Associated and Superior Scientific Researchers at the Argentine National Research Council (CONICET). J. Lavandera was a Fellow from the Argentine Scientific and Technologic Agency (ASTA). This work has been supported by grants from the CONICET, the ASTA and the University of Buenos Aires, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Dr. Susana Afonso
Rights and permissions
About this article
Cite this article
Lavandera, J.V., Batlle, A.M.D.C. & Buzaleh, A.M. Metabolization of Porphyrinogenic Agents in Brain: Involvement of the Phase I Drug Metabolizing System. A Comparative Study in Liver and Kidney. Cell Mol Neurobiol 27, 717–729 (2007). https://doi.org/10.1007/s10571-007-9154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9154-0