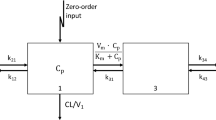
Abstract
Trabectedin (YondelisTM, formerly ET-743) is an anti-cancer drug currently undergoing phase II development. Despite extensive pharmacokinetic studies, the human disposition and excretory pathways of trabectedin remain largely unknown. Our objective was to determine the mass balance of trabectedin in humans. To this aim, we intravenously administered [14C]trabectedin to 8 cancer patients, followed by collection of whole blood, urine and faeces samples. A 24-h infusion was administered to 2 patients, whereas the other 6 patients received a 3-h infusion. Levels of total radioactivity and unchanged trabectedin were determined and used for calculation of pharmacokinetic parameters. No schedule dependency of pharmacokinetic parameters was observed apart from Cmax. Plasma and whole blood concentrations of [14C]trabectedin related radioactivity were comparable. Only 8% of the plasma exposure to [14C]trabectedin related compounds is accounted for by trabectedin, indicating the importance of metabolism in trabectedin elimination. Trabectedin displays a large volume of distribution (±1700 L), relative to total radioactivity (±220 L). [14C]trabectedin related radioactivity is mainly excreted in the faeces (mean: 55.5% of the dose). Urinary excretion accounts for 5.9% of the dose on average resulting in a mean overall recovery of 61.4% (3-h administration schedule). The excretion of unchanged trabectedin is very low both in faeces and in urine (< 1% of dose). In conclusion, trabectedin is extensively metabolised and principally excreted in the faeces.
Similar content being viewed by others
Abbreviations
- AUC0-96:
-
area under the plasma concentration curve extrapolated from 0–96 h
- AUCinf:
-
area under the plasma concentration curve extrapolated to infinity
- Cl:
-
total body clearance
- C max :
-
maximum plasma concentration
- LC-MS/MS:
-
liquid chromatography with tandem mass spectrometry
- LSC:
-
liquid scintillation counting
- t1/2:
-
terminal half-life
- t max :
-
time of maximum plasma concentration
- TRA:
-
total radioactivity
- ULN:
-
upper limit of normal
- V ss :
-
volume of distribution at steady state
References
Blay JY, Le Cesne A, Verweij J, Scurr M, Seynaeve C, Bonvalot S, Hogendoorn P, Jimeno J, Evrard V, Van Glabbeke M, Judson I: A phase II study of ET-743/trabectedin (‘Yondelis’) for patients with advanced gastrointestinal stromal tumours. Eur J Cancer 40: 1327–1331, 2004
Casanova M, Casali PG, Sessa C, et al.: Phase II study of 3-hour infusion of ET-743 in pretreated adult and pediatric patients with advanced/recurrent sarcomas-preliminary results from the Sendo and Italian Sarcoma Group (Abstract). Med Pediatr Oncol 39: 257, 2002
Dileo P, Casali PG, Bacci G, et al.: Phase II evaluation of 3-hr infusion ET-743 in patients with recurrent sarcomas (Abstract). Proc Am Soc Clin Oncol 21: 408a, 2002
Garcia-Carbonero R, Supko JG, Manola J, Seiden MV, Harmon D, Ryan DP, Quigley MT, Merriam P, Canniff J, Goss G, Matulonis U, Maki RG, Lopez T, Puchalski TA, Sancho MA, Gomez J, Guzman C, Jimeno J, Demetri GD: Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22: 1480-1490, 2004
George S, Maki RG, Harmon D, Supko J, Morgan J, Seiden M, Buonanno M, Griffin J, Moutsiolis A, Lopez T, Guzman C, Jimeno J, Demetri GD: Phase II study of ecteinascidin-743 (ET-743) given by 3-hour IV infusion in patients (pts) with soft tissue sarcomas (STS) failing prior chemotherapies (Abstract). Proc Am Soc Clin Oncol 21: 408a, 2002
Laverdiere C, Kolb EA, Supko JG, Gorlick R, Meyers PA, Maki RG, Wexler L, Demetri GD, Healey JH, Huvos AG, Goorin AM, Bagatell R, Ruiz-Casado A, Guzman C, Jimeno J, Harmon D: Phase II study of ecteinascidin 743 in heavily pretreated patients with recurrent osteosarcoma. Cancer 98: 832–840, 2003
Laverdiere C, Kolb A, Meyers P, Gorlick R, Maki RG, Wexler L, Demetri GD, Goorin A, Harmon D, Jimeno J, Guzman C, Bagatell R: Phase II study of ET-743 in recurrent osteosarcoma (Abstract). Proc Am Soc Clin Oncol 21: 96a, 2002
Le Cesne A: Phase II study of ET-743 in advanced soft tissue sarcoma (ASTS) in adult: a STBSG-EORTC trial (Abstract). Proc Am Soc Clin Oncol 19: 554a, 2000
Ryan DP, Puchalski T, Supko JG, Harmon D, Maki R, Garcia-Carbonero R, Kuhlman C, Winkelman J, Merriam P, Quigley T, Jimeno J, Manola J, Demetri GD: A phase II and pharmacokinetic study of ecteinascidin 743 in patients with gastrointestinal stromal tumors. Oncologist 7: 531–538, 2002
Sessa C, Colombo N, Bauer J, Perotti A, Curigliano G, Noberasco C, Capri G, Jimeno J, Marsoni S, Gianni L: Phase II study of salvage ET-743 given as 3-hr infusion in ovarian cancer (oc) patients (Abstract). Ann Oncol 13: 109, 2002
Moore BM, Seaman FC, Wheelhouse RT, Hurley LH: Mechanism for the catalytic activation of ecteinscidin 743 and its subsequent alkylation of guanine N2. J Am Chem Soc 120: 2490–2491, 1998
Pommier Y, Kohlhagen G, Bailly C, Waring M, Mazumder A, Kohn KW: DNA sequence-and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the Caribbean tunicate Ecteinascidia turbinata. Biochemistry 35: 13303–13309, 1996
Kanzaki A, Takebayashi Y, Ren XQ, Miyashita H, Mori S, Akiyama S, Pommier Y: Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol Cancer Ther 1: 1327–1334, 2002
Minuzzo M, Marchini S, Broggini M, Faircloth G, D’Incalci M, Mantovani R: Interference of transcriptional activation by the antineoplastic drug ecteinascidin-743. Proc Natl Acad Sci USA 97: 6780–6784, 2000
Jin S, Gorfajn B, Faircloth G, Scotto KW: Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc Natl Acad Sci USA 97: 6775–6779, 2000
D’Incalci M, Jimeno J: Preclinical and clinical results with the natural marine product ET-743. Expert Opin Investig Drugs 12: 1843–1853, 2003
van Kesteren C, de Vooght MM, Lopez-Lazaro L, Mathot RA, Schellens JHM, Jimeno JM, Beijnen JH: Yondelis (trabectedin, ET-743): The development of an anticancer agent of marine origin. Anticancer Drugs 14: 487–502, 2003
Villalona-Calero MA, Eckhardt SG, Weiss G, Hidalgo M, Beijnen JH, van Kesteren C, Rosing H, Campbell E, Kraynak M, Lopez-Lazaro L, Guzman C, Von Hoff DD, Jimeno J, Rowinsky EK: A phase I and pharmacokinetic study of ecteinascidin-743 on a daily × 5 schedule in patients with solid malignancies. Clin Cancer Res 8: 75–85, 2002
Sparidans RW, Rosing H, Hillebrand MJ, Lopez-Lazaro L, Jimeno JM, Manzanares I, van Kesteren C, Cvitkovic E, van Oosterom AT, Schellens JHM, Beijnen JH: Search for metabolites of ecteinascidin 743, a novel, marine-derived, anti-cancer agent, in man. Anticancer Drugs 12: 653–666, 2001
European Pharmacopoeia. Council of Europe, Strasbourg, 1997
Rosing H, Hillebrand MJ, Jimeno JM, Gomez A, Floriano P, Faircloth G, Henrar RE, Vermorken JB, Cvitkovic E, Bult A, Beijnen JH: Quantitative determination of Ecteinascidin 743 in human plasma by miniaturized high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Mass Spectrom 33: 1134–1140, 1998
Rowland M, Tozer TN: Clinical pharmacokinetics—concepts and applications. Williams & Wilkins, 1995
Schwartz DE, Bollag W, Obrecht P: Distribution and excretion studies of procarbazine in animals and man. Arzneimittelforschung 17: 1389–1393, 1967
DeVita VT, Denham C, Davidson JD, Oliverio VT: The physiological disposition of the carcinostatic 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in man and animals. Clin Pharmacol Ther 8: 566–577, 1967
Chaudhuri NK, Montag BJ, Heidelberger C: Studies on fluorinated pyrimidines. III. The metabolism of 5-fluorouracil-2-C14 and 5-fluoroorotic-2-C14 acid in vivo. Cancer Res 18: 318–328, 1958
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beumer, J.H., Rademaker-Lakhai, J.M., Rosing, H. et al. Trabectedin (YondelisTM, formerly ET-743), a mass balance study in patients with advanced cancer. Invest New Drugs 23, 429–436 (2005). https://doi.org/10.1007/s10637-005-2902-4
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-2902-4