Summary
Indisulam (N-(3-chloro-7-indolyl)-1,4-benzenedisulfonamide, GOAL, E7070) is a novel anti-cancer drug currently in phase II clinical development for the treatment of solid tumors. Phase I dose-escalation studies were conducted comparing four treatment schedules. Neutropenia and thrombocytopenia were dose limiting in all schedules. The aim of this study was to describe the extent and the time course of the hematological toxicity and its possible schedule dependency using a semi-physiological model.
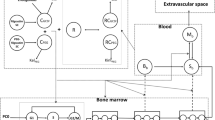
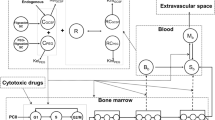
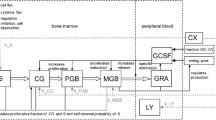
Data from 142 patients were analyzed using NONMEM. The semi-physiological model comprised a progenitor blood cell compartment, linked to the central circulation compartment, through 3 transition compartments representing the maturation chain in the bone marrow. Plasma concentrations of the drug were assumed to reduce the proliferation rate in the progenitor compartment according to a linear function. A feedback mechanism was included in the model representing the rebound effect of endogenous growth factors. The model was validated using a posterior predictive check.
The model adequately described the extent and time course of neutropenia and thrombocytopenia. The mean transition time (MTT, i.e. maturation time in bone marrow) of neutrophils was increased by 47% in patients who received indisulam as a weekly dose administered for four out of every six weeks. For platelets, MTT was increased by 33% in patients who received this schedule and also in patients who received a continuous 120-h infusion. The validation procedure indicated that the model adequately predicts the nadir value of neutrophils and platelets and the time to reach this nadir.
A semi-physiological model was successfully applied to describe the time course and extent of the neutropenia and thrombocytopenia after indisulam administration for four treatment schedules.
Similar content being viewed by others
References
Owa T, Okauchi T, Yoshimatsu K, Sugi NH, Ozawa Y, Nagasu T, Koyanagi N, Okabe T, Kitoh K, Yoshino H: A focused compound library of novel N-(7-indolyl)benzenesulfonamides for the discovery of potent cell cycle inhibitors. Bioorg Med Chem Lett 10: 1223–1226, 2000
Owa T, Yoshino H, Okauchi T, Yoshimatsu K, Ozawa Y, Sugi NH, Nagasu T, Koyanagi N, Kitoh K: Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem 42: 3789–3799, 1999
Ozawa Y, Sugi NH, Nagasu T, Owa T, Watanabe T, Koyanagi N, Yoshino H, Kitoh K, Yoshimatsu K: E7070, a novel sulphonamide agent with potent antitumour activity in vitro and in vivo. Eur J Cancer 37: 2275–2282, 2001
Fukuoka K, Usuda J, Iwamoto Y, Fukumoto H, Nakamura T, Yoneda T, Narita N, Saijo N, Nishio K: Mechanisms of action of the novel sulfonamide anticancer agent E7070 on cell cycle progression in human non-small cell lung cancer cells. Invest New Drugs 19: 219–227, 2001
Oda Y, Owa T, Sato T, Boucher B, Daniels S, Yamanaka H, Shinohara Y, Yokoi A, Kuromitsu J, Nagasu T: Quantitative chemical proteomics for identifying candidate drug targets. Anal Chem 75: 2159–2165, 2003
Raymond E, ten Bokkel Huinink WW, Taieb J, Beijnen JH, Faivre S, Wanders J, Ravic M, Fumoleau P, Armand JP, Schellens JHM: Phase I and pharmacokinetic study of E7070, a novel chloroindolyl sulfonamide cell-cycle inhibitor, administered as a one-hour infusion every three weeks in patients with advanced cancer. J Clin Oncol 20: 3508–3521, 2002
Punt CJ, Fumoleau P, van de WB, Faber MN, Ravic M, Campone M: Phase I and pharmacokinetic study of E7070, a novel sulfonamide, given at a daily times five schedule in patients with solid tumors. A study by the EORTC-early clinical studies group (ECSG). Ann Oncol 12: 1289–1293, 2001
Terret C, Zanetta S, Roche H, Schellens JH, Faber MN, Wanders J, Ravic M, Droz JP: Phase I clinical and pharmacokinetic study of E7070, a novel sulfonamide given as a 5-day continuous infusion repeated every 3 weeks in patients with solid tumours. A study by the EORTC Early Clinical Study Group (ECSG). Eur J Cancer 39: 1097–1104, 2003
Dittrich C, Dumez H, Calvert H, Hanauske A, Faber M, Wanders J, Yule M, Ravic M, Fumoleau P: Phase I and pharmacokinetic study of E7070, a chloroindolyl-sulfonamide anticancer agent, administered on a weekly schedule to patients with solid tumors. Clin Cancer Res 9: 5195–5204, 2003
Raymond E, Fumoleau P, Roche H, Schellens JHM, Dittrich C, Punt CJA, Droz JP, Calvert AH, van Oosterom A, Wanders J, Ravic M: Combined results of 4 phase I and pharmacokinetic studies of E7070, a novel chloroindoly-sulphonamide inhibiting the activation of cdk2 and cyclin E. Clin Cancer Res 6: 4529s–2000
Van Kesteren Ch, Mathot RA, Raymond E, Armand JP, Dittrich C, Dumez H, Roche H, Droz JP, Punt C, Ravic M, Wanders J, Beijnen JH, Fumoleau P, Schellens JHM: Population pharmacokinetics of the novel anticancer agent E7070 during four phase I studies: model building and validation. J Clin Oncol 20: 4065–4073, 2002
van Kesteren Ch, Dittrich C, Dumez H, Roche H, Droz JP, Punt CJA, Ravic M, Wanders J, Beijnen JH, Fumoleau P, Schellens JHM: Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of the novel anticancer agent E7070 in four phase I studies (Abstract). Br J Clin Pharmcol 53: 553P–2002
Minami H, Sasaki Y, Saijo N, Ohtsu T, Fujii H, Igarashi T, Itoh K: Indirect-response model for the time course of leukopenia with anticancer drugs. Clin Pharmacol Ther 64: 511–521, 1998
Karlsson MO, Port RE, Ratain MJ, Sheiner LB: A population model for the leukopenic effect of etoposide. Clin Pharmacol Ther 57: 325–334, 1995
Friberg LE, Brindley CJ, Karlsson MO, Devlin AJ: Models of schedule dependent haematological toxicity of 2′-deoxy-2′-methylidenecytidine (DMDC). Eur J Clin Pharmacol 56: 567–574, 2000
Friberg LE, Freijs A, Sandstrom M, Karlsson MO: Semiphysiological model for the time course of leukocytes after varying schedules of 5-fluorouracil in rats. J Pharmacol Exp Ther 295: 734–740, 2000
Zamboni WC, D’Argenio DZ, Stewart CF, MacVittie T, Delauter BJ, Farese AM, Potter DM, Kubat NM, Tubergen D, Egorin MJ: Pharmacodynamic model of topotecan-induced time course of neutropenia. Clin Cancer Res 7: 2301–2308, 2001
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO: Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20: 4713–4721, 2002
Beal SL, Boeckman AJ, Sheiner LB: NONMEM User’s Guides.University of California at San Francisco, San Francisco, CA (1988–1992
Ette EI, Ludden TM: Population pharmacokinetic modeling: The importance of informative graphics. Pharm Res 12: 1845–1855, 1995
Ette EI: Statistical graphics in pharmacokinetics and pharmacodynamics: A tutorial. Ann Pharmacother 32: 818–828, 1998
Jonsson EN, Karlsson MO: Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58: 51–64, 1999
Yano Y, Beal SL, Sheiner LB: Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28: 171–192, 2001
Jonsson EN, Karlsson MO: Automated covariate model building within NONMEM. Pharm Res 15: 1463–1468, 1998
Price TH, Chatta GS, Dale DC: Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 88: 335–340, 1996
Van Warmerdam LJC, Van den Bemt BJF, ten Bokkel Huinink WW, Maes RAA, Beijnen JH: Dose individualisation in cancer chemotherapy: Pharmacokinetic and pharmacodynamic relationships. Cancer Res Ther Control 4: 277–291, 1995
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the New Drug Development Group of the EORTC.
Rights and permissions
About this article
Cite this article
van Kesteren, C., Zandvliet, A.S., Karlsson, M.O. et al. Semi-physiological model describing the hematological toxicity of the anti-cancer agent indisulam. Invest New Drugs 23, 225–234 (2005). https://doi.org/10.1007/s10637-005-6730-3
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-6730-3