Summary
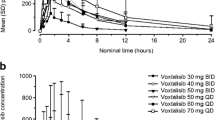
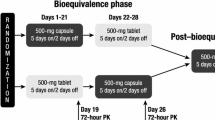
Purpose This phase I, open-label, randomized, 2-part crossover study assessed the safety, pharmacokinetics and relative bioavailability of single doses of the anticancer MET inhibitor foretinib (formerly known as GSK1363089, EXEL-2880 and XL-880) free base tablet formulation compared to a bisphosphate salt capsule formulation (Part 1), and assessed the safety, efficacy, and pharmacokinetics of the bisphosphate salt capsule administered 3 times a week in cancer patients (Part 2). Patients and Methods In Part 1, patients were randomized in a crossover manner to receive a single oral dose of foretinib formulated as a bisphosphate salt capsule (240 mg; 183 mg free base equivalent) followed one week later by a single dose of a free base tablet (180 mg), or vice versa where the treatment sequence was reversed. In Part 2, patients self-administered oral doses of bisphosphate salt capsules (200 mg) 3 times a week until disease progression. Results Twelve patients with solid tumors were enrolled and completed Part 1, and 10 patients continued into Part 2. Most AEs were mild or moderate in severity. The most common drug-related AEs were fatigue, diarrhea, and nausea. The least-squares (LS) mean total area under the curve was 3144 and 3514 ng*h/mL for the free base tablet and bisphosphate salt capsule, respectively, with a ratio of 0.89 (90% confidence interval, CI: 0.69, 1.16). The LS mean maximal concentration (Cmax) was 81.6 and 98.5 ng/mL for the free base and bisphosphate salt, respectively, with a ratio of 0.83 (90% confidence interval, CI: 0.67, 1.02). The time to reach Cmax was ∼4 h for both formulations. The pharmacokinetics of foretinib were not clinically different between the 2 formulations. Of the 10 patients assessed for efficacy, 3 patients achieved stable disease. Conclusions Foretinib was well tolerated as single doses of both the free base and bisphosphate salt formulations. The pharmacokinetics and relative bioavailability of the 2 formulations were not clinically different. The bisphosphate salt formulation was well tolerated on a 3-times a week dosing schedule, and reached steady-state plasma concentration after 2 weeks.

Similar content being viewed by others
References
Qian F, Engst S, Yamaguchi K et al (2009) Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res 69:8009–8016
Bean J, Brennan C, Shih J-Y et al (2007) MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104:20932–20937
Comoglio PM, Boccaccio C (1996) The HGF receptor family: unconventional signal transducers for invasive cell growth. Genes Cells 1:347–354
Cui JJ (2007) Inhibitors targeting hepatocyte growth factor receptor and their potential therapeutic applications. Expert Opin Ther Patents 17:1035–1045
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) MET, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Ma PC, Kijima T, Maulik G et al (2003) c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 63:6272–6281
Eder JP, Vande Woude GF, Boerner SA, LoRusso PM (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15:2207–2214
Benvenuti S, Comoglio PM (2007) The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 213:316–325
Holmes K, Roberts OL, Thomas AL, Cross MJ (2007) Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 19:2003–2012
Bottaro DP, Liotta LA (2003) Out of air is not out of action. Nature 423:593–595
Xin X, Yang S, Ingle G et al (2001) Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 158:1111–1120
Kim ES, Salgia R (2009) MET pathway as a therapeutic target. J Thorac Oncol 4:444–447
Rini BI (2007) Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res 13:1098–1106
Eder JP, Shapiro GI, Appleman LJ et al (2010) A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res 16(13):3507–3516
LoRusso P, Eder JP, Sherman L et al (2009) Angiogenesis and Antiangiogenesis Agents: Final results of a phase I dose escalation study of the safety and pharmacokinetics of foretinib administered orally daily to patients with solid tumors. Mol Cancer Ther 8(12 Suppl):A8
Jhawer M, Kindler H, Wainberg Z et al (2009) Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. Journal of Clinical Oncology, 2009 ASCO Annual Meeting Proceedings Post-Meeting Edition Vol 27, No 15 S (May 20 Supplement):4502
Srinivasan R, Linehan WM, Vaishampayan U et al (2009) A phase II study of two dosing regimens of GSK 1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in patients (pts) with papillary renal carcinoma (PRC). Journal of Clinical Oncology, 2009 ASCO Annual Meeting Proceedings Post-Meeting Edition Vol 27 (15 S) (May 20 Supplement), 5103
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2:205–216
Acknowledgements
We would like to acknowledge Ann Wintervann for assistance with preparation on the manuscript and Lei Fang for assistance with statistical analysis. GSK provided funding for this study and for editorial support (copyediting, fact checking, referencing) by Publication Connexion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Joseph F. Kleha, Kevin H. Laubscher, Laurel M. Adams, Peter L. Bonate, Colleen Fitzgerald, Steven Weller, and Yanmei Xu are employed by GlaxoSmithKline.
Rights and permissions
About this article
Cite this article
Naing, A., Kurzrock, R., Adams, L.M. et al. A Comparison of the pharmacokinetics of the anticancer MET inhibitor foretinib free base tablet formulation to bisphosphate salt capsule formulation in patients with solid tumors. Invest New Drugs 30, 327–334 (2012). https://doi.org/10.1007/s10637-010-9536-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9536-x