Abstract
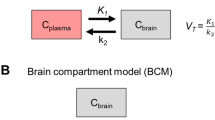
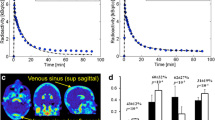
Positron emissiontomography (PET) with the drug radiolabelled allows a direct measurement of brain or other organ kinetics, information which can be essential in drug development. Usually, however, a PET-tracer is administered intravenously (i.v.), whereas the therapeutic drug is mostly given orally or by a different route to the PET-tracer. In such cases, a recalculation is needed to make the PET data representative for the alternative administration route. To investigate the blood–brain barrier penetration of a drug (zolmitriptan) using dynamic PET and by PK modelling quantify the brain concentration of the drug after the nasal administration of a therapeutic dose. [11C]Zolmitriptan at tracer dose was administered as a short i.v. infusion and the brain tissue and venous blood kinetics of [11C]zolmitriptan was measured by PET in 7 healthy volunteers. One PET study was performed before and one 30 min after the administration of 5 mg zolmitriptan as nasal spray. At each of the instances, the brain radioactivity concentration after subtraction of the vascular component was determined up to 90 min after administration and compared to venous plasma radioactivity concentration after correction for radiolabelled metabolites. Convolution methods were used to describe the relationship between arterial and venous tracer concentrations, respectively between brain and arterial tracer concentration. Finally, the impulse response functions derived from the PET studies were applied on plasma PK data to estimate the brain zolmitriptan concentration after a nasal administration of a therapeutic dose. The studies shows that the PET data on brain kinetics could well be described as the convolution of venous tracer kinetics with an impulse response including terms for arterial-to-venous plasma and arterial-to-brain impulse responses. Application of the PET derived impulse responses on the plasma PK from nasal administration demonstrated that brain PK of zolmitriptan increased with time, achieving about 0.5 mg/ml at 30 min and close to a maximum of 1.5 mg/ml after 2 hr. A significant brain concentration was observed already after 5 min. The data support the notation of a rapid brain availability of zolmitriptan after nasal administration
Similar content being viewed by others
References
Bergstrom M., Cass L.M., Valind S., Westerberg G., Lundberg E.L., Gray S., Bye A., Langstrom B. (1999). Deposition and disposition of [11C]zanamivir following administration as an intranasal spray. Evaluation with positron emission tomograph. Clin. Pharmacokinet. 36:33–39
Gefvert O., Bergstrom M., Langstrom B., Lundberg T., Lindstrom L., Yates R. (1998). Time course of central nervous dopamine-D2 and 5-HT2 receptor blockade and plasma drug concentrations after discontinuation of quetiapine (Seroquel) in patients with schizophrenia. Psychopharmacology (Berl) 135:119–126
Gefvert O., Lundberg T., Wieselgren I.M., Bergstrom M., Langstrom B., Wiesel F., Lindstrom L. (2001). D(2) and 5HT(2A) receptor occupancy of different doses of quetiapine in schizophrenia: A PET study. Eur Neuropsychopharm. 11:105–110
Hartvig P., Bergstrom M., Antoni G., Langstrom B. (2002). Positron emission tomography and brain monoamine neurotransmission–entries for study of drug interactions. Curr. Pharm. Des. 8:1417–1434
Kihlberg T., Karimi F., Langstrom B. (2002). [(11)C] Carbon monoxide in selenium-mediated synthesis of (11)C-carbamoyl compounds. J. Org. Chem. 67:3687–3692
Learned-Coughlin S.M., Bergstrom M., Savitcheva I., Ascher J., Schmith V.D., Langstrom B. (2003). In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol. Psychiatry. 54:800–805
Lesko L.J., Rowland M., Peck C.C., Blaschke T.F. (2000). Optimizing the science of drug development: Opportunities for better candidate selection and accelerated evaluation in humans. Pharm. Res. 17:1335–1344
Peck C.C., Barr W.H., Benet L.Z., Collins J., Desjardins R.E., Furst D.E., Harter J.G., Levy G., Ludden T., Rodman J.H. et al. (1992). Opportunities for integration of pharmacokinetics, pharmacodynamics, and toxicokinetics in rational drug development. J. Pharm. Sci. 81:605–610
Lammertsma A.A. (2002). Radioligand studies: Imaging and quantitative analysis. Eur. Neuropsychopharm. 12:513–516
Matarrese M., Salimbeni A., Turolla E.A., Turozzi D., Moresco R.M., Poma D., Magni F., Todde S., Rossetti C., Sciarrone M.T., Bianchi G., Kienle M.G., Fazio F. (2004). 11C-Radiosynthesis and preliminary human evaluation of the disposition of the ACE inhibitor [11C]zofenoprilat. Bioorg. Med. Chem. 12:603–611
Hutchinson O.C., Collingridge D.R., Barthel H., Price P.M., Aboagye E.O. (2003). Pharmacodynamics of radiolabelled anticancer drugs for positron emission tomography. Curr. Pharm. Des. 9:931–944
Propper D.J., de Bono J., Saleem A., Ellard S., Flanagan E., Paul J., Ganesan T.S., Talbot D.C., Aboagye E.O., Price P., Harris A.L., Twelves C. (2003). Use of positron emission tomography in pharmacokinetic studies to investigate therapeutic advantage in a phase I study of 120-hour intravenous infusion XR5000. J. Clin. Oncol. 21:203–210
Gulyas B., Halldin C., Sandell J., Karlsson P., Sovago J., Karpati E., Kiss B., Vas A., Cselenyi Z., Farde L. (2002). PET studies on the brain uptake and regional distribution of [11C] vinpocetine in human subjects. Acta. Neurol. Scand. 106:325–332
Bergstrom M., Grahnen A., Langstrom B. (2003). Positron emission tomography microdosing: A new concept with application in tracer and early clinical drug developmet. Eur. J. Clin. Pharmacol. 59:357–366
Bergstrom M., Nordberg A., Lunell E., Antoni G., Langstrom B. (1995). Regional deposition of inhaled 11C-nicotine vapor in the human airway as visualized by positron emission tomography. Clin. Pharmacol. Ther. 57:309–317
Uemura N., Charlesworth B.R., Onishi T., Mitaniyama A., Kaneko T., Ninomiya K., Nakamura K., Tateno M. (2003). Zomitriptan is detectable in plasma as early as 2 to 5 minutes after administration by nasal spray. Ann. Meeting Prog. Abstr. Headache: J. Head Face Pain 43:509–592, S159
M. Kågedahl, T. Duvauchelle, L. Hovesepian, B. R. Charlesworth, H.-L. Su, and R. A. Yates. Zolmitriptan demonstrates good pharmacokinetic consistency between and within individuals following intranasal administration. pA2 [serial online] 1: abstract 211P. Available from http://www.pa2online.org/ (2003).
Gawel M., Aschoff J., May A., Charlesworth B.R. Zolmitriptan Nasal Spray: Efficacy, Onset of Action and Patient Satisfaction in Phases I and II of the REALIZE Study. Ann. Meeting Prog. Abstr. Headache: J. Head Face Pain 44:457–536, F23 (2004).
Wall A., Kagedal M., Bergstrom M., Jacobsson E., Nilsson D., Antoni G., Frandberg P., Gustavsson S-A., Langstrom B., Yates R. (2005). Distribution of Zolmitriptan into the CNS in healthy volunteers. A positron emission tomography study. Drugs R D 6:139–147
Ebling W.F., Wada D.R., Stanski D.R. (1994). From piecewise to full physiologic pharmacokinetic modeling: Applied to thiopental disposition in the rat. J. Pharmacokinet. Biopharm. 22:259–292
Verotta D. (1989). An inequality-constrained least-squares deconvolution method. J. Pharmacokinet. Biopharm. 17:269–289
Pitsiu M., Gries J.M., Benowitz N., Gourlay S.G., Verotta D. (2002). Modeling nicotine arterial-venous differences to predict arterial concentrations and input based on venous measurements: Application to smokeless tobacco and nicotine gum. J. Pharmacokinet. Pharmacodyn. 29:383–402
Gunn R.N., Gunn S.R., Cunningham V.J. (2001). Positron emission tomography compartmental models. J. Cereb. Blood Flow Metab. 21:635–652
Gunn R.N., Gunn S.R., Turkheimer F.E., Aston J.A., Cunningham V.J. (2002). Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. J. Cereb. Blood Flow Metab. 22:1425–1439
Cunningham V.J., Jones T. (1993). Spectral analysis of dynamic PET studies. J. Cereb. Blood Flow Metab. 13:15–23
O’Hara T., Hayes S., Davis J., Devane J., Smart T., Dunne A. (2001). In vivo-in vitro correlation (IVIVC) modeling incorporating a convolution step. J. Pharmacokinet. Pharmacodyn. 28:277–298
Gomeni R., Dangeli C., Bye A. (2002). In silico prediction of optimal in vivo delivery properties using convolution-based model and clinical trial simulation. Pharm. Res. 19:99–103
Chiou W.L., Lam G., Chen M.L., Lee M.G. (1981). Arterial-venous plasma concentration differences of six drugs in the dog and rabbit after intravenous administration. Res. Commun. Chem. Pathol. Pharmacol. 32:27–39
Porchet H.C., Benowitz N.L., Sheiner L.B., Copeland J.R. (1987). Apparent tolerance to the acute effect of nicotine results in part from distribution kinetics. J. Clin. Invest. 80:1466–1471
Tuk B., Herben V.M., Mandema J.W., Danhof M. (1998). Relevance of arteriovenous concentration differences in pharmacokinetic–pharmacodynamic modeling of midazolam. J. Pharmacol. Exp. Ther. 284:202–207
Hirvonen J., Nagren K., Kajander J., Hietala J. (2001). Measurement of cortical dopamine d1 receptor binding with 11C[SCH23390]: A test-retest analysis. J. Cereb. Blood Flow Metab. 21:1133–1145
Vilkman H., Kajander J., Nagren K., Oikonen V., Syvalahti E., Hietala J. (2000). Measurement of extrastriatal D2-like receptor binding with [11C]FLB 457–A test-retest analysis. Eur. J. Nucl. Med. 27:1666–1673
Hietala J., Nagren K., Lehikoinen P., Ruotsalainen U., Syvalahti E. (1999). Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: A test-retest analysis. J. Cereb. Blood Flow Metab. 19:210–217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergström, M., Yates, R., Wall, A. et al. Blood–Brain Barrier Penetration of Zolmitriptan—Modelling of Positron Emission Tomography Data. J Pharmacokinet Pharmacodyn 33, 75–91 (2006). https://doi.org/10.1007/s10928-005-9001-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-005-9001-1