Abstract
Purpose
This study was aimed to investigate the effects of a phenolic antioxidant, butylated hydroxyanisole (BHA) and its metabolite, tert-butylhydroquinone (tBHQ) on the induction of HO-1, NQO1 and Nrf2 proteins and their regulatory mechanisms in primary-cultured hepatocytes.
Methods
After exposure of BHA and tBHQ to primary-cultured rat and human hepatocytes and mouse neonatal fibroblasts (MFs), Western blot, semi-quantitative RT-PCR and microarray analysis were conducted.
Results
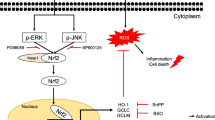
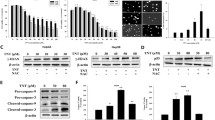
Induction of HO-1, NQO1 and Nrf2 proteins and activation of ERK1/2 and JNK1/2 were observed after BHA and tBHQ treatments in primary-cultured rat and human hepatocytes. Semi-quantitative RT-PCR study and microarray analysis revealed that HO-1 and NQO1 were transcriptionally activated in primary-cultured rat hepatocytes and a substantial transcriptional activation, including HO-1 occurred in primary-cultured human hepatocytes after BHA treatment. Whereas BHA failed to induce HO-1 in wild-type and Nrf2 knock-out MFs, tBHQ strongly induced HO-1 in wild-type, but not in Nrf2 knock-out MFs.
Conclusions
Our data demonstrate that both BHA and tBHQ are strong chemical inducers of HO-1, NQO1 and Nrf2 proteins in primary-cultured human and rat hepatocytes with the activation of MAPK ERK1/2 and JNK1/2. However, in MFs, BHA failed to induce HO-1, whereas tBHQ strongly induced HO-1 in Nrf2 wild-type but not in Nrf2 knock-out, suggesting that Nrf2 is indispensable for tBHQ-induced HO-1 in MF.





Similar content being viewed by others
References
J. S. Lee and Y. J. Surh. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett.224:171–184 (2005).
C. Chen and A. N. Kong. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic. Biol. Med.36:1505–1516 (2004).
K. Itoh, T. Chiba, S. Takahashi, T. Ishii, K. Igarashi, Y. Katoh, T. Oyake, N. Hayashi, K. Satoh, I. Hatayama, M. Yamamoto, and Y. Nabeshima. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun.236:313–322 (1997).
Y. J. Surh. Cancer chemoprevention with dietary phytochemicals. Nat. Rev., Cancer. 3:768–780 (2003).
L. E. Otterbein, M. P. Soares, K. Yamashita, and F. H. Bach. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol.24:449–455 (2003).
K. A. Kirkby and C. A. Adin. Products of heme oxygenase and their potential therapeutic applications. Am. J. Physiol., Renal Physiol.290:F563–F571 (2006).
Z. Dong, Y. Lavrovsky, M. A. Venkatachalam, and A. K. Roy. Heme oxygenase-1 in tissue pathology: the yin and yang. Am. J. Pathol.156:1485–1488 (2000).
D. E. Baranano, M. Rao, C. D. Ferris, and S. H. Snyder. Biliverdin reductase: a major physiologic cytoprotectant. Proc. Natl. Acad. Sci. U. S. A.99:16093–16098 (2002).
L. E. Otterbein, F. H. Bach, J. Alam, M. Soares, H. Tao Lu, M. Wysk, R. J. Davis, R. A. Flavell, and A. M. Choi. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med.6:422–428 (2000).
R. Foresti, J. Hammad, J. E. Clark, T. R. Johnson, B. E. Mann, A. Friebe, C. J. Green, and R. Motterlini. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br. J. Pharmacol.142:453–460 (2004).
H. O. Pae, G. S. Oh, B. M. Choi, S. C. Chae, Y. M. Kim, K. R. Chung, and H. T. Chung. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol.172:4744–4751 (2004).
B. S. Zuckerbraun, T. R. Billiar, S. L. Otterbein, P. K. Kim, F. Liu, A. M. Choi, F. H. Bach, and L. E. Otterbein. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J. Exp. Med.198:1707–1716 (2003).
C. D. Ferris, S. R. Jaffrey, A. Sawa, M. Takahashi, S. D. Brady, R. K. Barrow, S. A. Tysoe, H. Wolosker, D. E. Baranano, S. Dore, K. D. Poss, and S. H. Snyder. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol.1:152–157 (1999).
P. Nioi and J. D. Hayes. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop–helix transcription factors. Mutat. Res.555:149–171 (2004).
D. Ross. Quinone reductases multitasking in the metabolic world. Drug Metab. Rev.36:639–654 (2004).
G. Asher, J. Lotem, B. Cohen, L. Sachs, and Y. Shaul. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc. Natl. Acad. Sci. U. S. A.98:1188–1193 (2001).
G. Asher, J. Lotem, L. Sachs, C. Kahana, and Y. Shaul. Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1. Proc. Natl. Acad. Sci. U. S. A.99:13125–13130 (2002).
L. W. Wattenberg. Inhibition of chemical carcinogen-induced pulmonary neoplasia by butylated hydroxyanisole. J. Natl. Cancer Inst.50:1541–1544 (1973).
B. S. Reddy, Y. Maeura, and J. H. Weisburger. Effect of various levels of dietary butylated hydroxyanisole on methylazoxymethanol acetate-induced colon carcinogenesis in CF1 mice. J. Natl. Cancer Inst.71:1299–1305 (1983).
F. L. Chung, M. Wang, S. G. Carmella, and S. S. Hecht. Effects of butylated hydroxyanisole on the tumorigenicity and metabolism of N-nitrosodimethylamine and N-nitrosopyrrolidine in A/J mice. Cancer Res.46:165–168 (1986).
G. M. Williams and M. J. Iatropoulos. Inhibition of the hepatocarcinogenicity of aflatoxin B1 in rats by low levels of the phenolic antioxidants butylated hydroxyanisole and butylated hydroxytoluene. Cancer Lett.104:49–53 (1996).
H. Verhagen, P. A. Schilderman, and J. C. Kleinjans. Butylated hydroxyanisole in perspective. Chem. Biol. Interact. 80:109–134 (1991).
H. Verhagen, H. H. Thijssen, F. ten Hoor, and J. C. Kleinjans. Disposition of single oral doses of butylated hydroxyanisole in man and rat. Food Chem. Toxicol.27:151–158 (1989).
L. W. Wattenberg, J. B. Coccia, and L. K. Lam. Inhibitory effects of phenolic compounds on benzo(a)pyrene-induced neoplasia. Cancer Res.40:2820–2823 (1980).
B. Hager, J. R. Bickenbach, and P. Fleckman. Long-term culture of murine epidermal keratinocytes. J. Invest. Dermatol.112:971–976 (1999).
G. Shen, C. Xu, R. Hu, M. R. Jain, S. Nair, W. Lin, C. S. Yang, J. Y. Chan, and A. N. Kong. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm. Res.22:1805–1820 (2005).
D. Stewart, E. Killeen, R. Naquin, S. Alam, and J. Alam. Degradation of transcription factor Nrf2 via the ubiquitin–proteasome pathway and stabilization by cadmium. J. Biol. Chem.278:2396–2402 (2003).
J. Alam, E. Killeen, P. Gong, R. Naquin, B. Hu, D. Stewart, J. R. Ingelfinger, and K. A. Nath. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am. J. Physiol., Renal Physiol.284:F743–F752 (2003).
Y. S. Keum, W. S. Jeong, and A. N. Kong. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res.555:191–202 (2004).
D. Ross, J. K. Kepa, S. L. Winski, H. D. Beall, A. Anwar, and D. Siegel. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 129:77–97 (2000).
W. S. Jeong, M. Jun, and A. N. Kong. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal.8:99–106 (2006).
S. R. Chinni and F. H. Sarkar. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin. Cancer Res.8:1228–1236 (2002).
M. Saleem, F. Afaq, V. M. Adhami, and H. Mukhtar. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene23:5203–5214 (2004).
K. H. Chun, J. W. Kosmeder II, S. Sun, J. M. Pezzuto, R. Lotan, W. K. Hong, and H. Y. Lee. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J. Natl. Cancer Inst.95:291–302 (2003).
R. Kahl, S. Weinke, and H. Kappus. Production of reactive oxygen species due to metabolic activation of butylated hydroxyanisole. Toxicology59:179–194 (1989).
P. A. Schilderman, J. M. van Maanen, E. J. Smeets, F. ten Hoor, and J. C. Kleinjans. Oxygen radical formation during prostaglandin H synthase-mediated biotransformation of butylated hydroxyanisole. Carcinogenesis14:347–353 (1993).
D. C. Thompson, Y. N. Cha, and M. A. Trush. The peroxidase-dependent activation of butylated hydroxyanisole and butylated hydroxytoluene (BHT) to reactive intermediates. Formation of BHT-quinone methide via a chemical–chemical interaction. J. Biol. Chem.264:3957–3965 (1989).
G. M. Williams, M. J. Iatropoulos, and J. Whysner. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol.37:1027–1038 (1999).
F. Iverson. In vivo studies on butylated hydroxyanisole. Food Chem. Toxicol.37:993–997 (1999).
R. Yu, S. Mandlekar, and A. T. Kong. Molecular mechanisms of butylated hydroxylanisole-induced toxicity: induction of apoptosis through direct release of cytochrome c. Mol. Pharmacol.58:431–437 (2000).
Acknowledgments
We thank all the members in Dr. Tony Kong's lab for the help in the discussion and preparation of this manuscript. This work was supported by the National Institute of Health Grant R01-CA094828.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keum, YS., Han, YH., Liew, C. et al. Induction of Heme Oxygenase-1 (HO-1) and NAD[P]H: Quinone Oxidoreductase 1 (NQO1) by a Phenolic Antioxidant, Butylated Hydroxyanisole (BHA) and Its Metabolite, tert-Butylhydroquinone (tBHQ) in Primary-Cultured Human and Rat Hepatocytes. Pharm Res 23, 2586–2594 (2006). https://doi.org/10.1007/s11095-006-9094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9094-2