Abstract
Purpose
Recently identified organic solute transporter (Ost) α and β are located on the basolateral membrane of enterocytes and may be responsible for the intestinal absorption of many substrates including bile acids. In the present study, the mechanism governing the transcriptional regulation of their expression was investigated.
Methods and Results
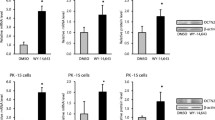
To clarify the transcriptional regulation of Osts, reporter gene assays were performed using mouse Ostα/β promoter-luciferase reporter constructs. Co-transfection of the constructs with farnesoid X receptor (FXR) and retinoid X receptor α (RXRα) or liver X receptor α (LXRα) and RXRα into Caco-2 cells induced the transcriptional activities of both Ost α and β and further increases were observed following treatment with each agonist. Sequence analyses indicated the presence of IR-1 regions in Ostα and Ostβ promoters, which was confirmed by the finding that the deletion of IR-1 sequences abolished the response to FXR and LXRα. Furthermore, mutations in IR-1 reduced the FXR- and LXRα-dependent transactivation of Ostα/β. Together with the detection of direct binding of FXR/RXRα and LXRα/RXRα to the IR-1 elements, the presence of functional FXRE/LXRE was revealed in the promoter region of both Ostα and Ostβ. In addition, the stimulatory effect of FXR/RXRα and LXRα/RXRα on Ostα, but not on Ostβ, was further enhanced by HNF-4α.
Conclusions
It was concluded that LXRα/RXRα transcriptionally regulate mouse Ostα/β via IR-1 elements shared with FXR/RXRα. Exposure to FXR/LXRα modulators may affect the disposition of Ostα/β substrates.








Similar content being viewed by others
Abbreviations
- ASBT:
-
apical sodium-dependent bile acid transporter
- BSEP:
-
bile salt export pump
- CDCA:
-
chenodeoxycholic acid
- EMSA:
-
electrophoretic mobility shift assay
- FXR:
-
farnesoid X receptor
- FXRE:
-
FXR/RXR binding element
- HNF-4:
-
hepatocyte nuclear factor-4
- I–BABP:
-
ileal–bile acid binding protein
- IR-1:
-
inverted repeat-1
- LXR:
-
liver X receptor
- LXRE:
-
LXR/RXR binding element
- OST:
-
organic solute transporter
- RXR:
-
retinoid X receptor
References
H. Suzuki, and Y. Sugiyama. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci 12:3–12 (2000).
H. Kusuhara, and Y. Sugiyama. Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J. Control. Release 78:43–54 (2002).
C. G. Dietrich, A. Geier, and R. P. Oude Elferink. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut 52:1788–1795 (2003).
Q. Mao, and J. D. Unadkat. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 7:E118–E133 (2005).
T. Okano, K. Inui, H. Maegawa, M. Takano, and R. Hori. H+ coupled uphill transport of aminocephalosporins via the dipeptide transport system in rabbit intestinal brush-border membranes. J. Biol. Chem. 261:14130–14134 (1986).
M. E. Ganapathy, M. Brandsch, P. D. Prasad, V. Ganapathy, and F. H. Leibach. Differential recognition of beta -lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J. Biol. Chem. 270:25672–25677 (1995).
D. Kobayashi, T. Nozawa, K. Imai, J. Nezu, A. Tsuji, and I. Tamai. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J. Pharmacol. Exp. Ther. 306:703–708 (2003).
H. Satoh, F. Yamashita, M. Tsujimoto, H. Murakami, N. Koyabu, H. Ohtani, and Y. Sawada. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab. Dispos. 33:518–523 (2005).
G. K. Dresser, D. G. Bailey, B. F. Leake, U. I. Schwarz, P. A. Dawson, D. J. Freeman, and R. B. Kim. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin. Pharmacol. Ther. 71:11–20 (2002).
G. K. Dresser, R. B. Kim, and D. G. Bailey. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin. Pharmacol. Ther. 77:170–177 (2005).
A. L. Craddock, M. W. Love, R. W. Daniel, L. C. Kirby, H. C. Walters, M. H. Wong, and P. A. Dawson. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 274:G157–G169 (1998).
M. Kida, Y. Mano, Y. Ueno, K. Kobayashi, J. Goto, M. Ishii, and T. Shimosegawa. Vectorial transport of bile acids in immortalized mouse bile duct cells. Hepatol. Res. 27:151–157 (2003).
T. Hirohashi, H. Suzuki, H. Takikawa, and Y. Sugiyama. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J. Biol. Chem. 275:2905–2910 (2000).
D. Rost, S. Mahner, Y. Sugiyama, and W. Stremmel. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am. J. Physiol.: Gastrointest. Liver Physiol. 282:G720–G726 (2002).
T. Shoji, H. Suzuki, H. Kusuhara, Y. Watanabe, S. Sakamoto, and Y. Sugiyama. ATP-dependent transport of organic anions into isolated basolateral membrane vesicles from rat intestine. Am. J. Physiol.: Gastrointest. Liver Physiol. 287:G749–G756 (2004).
W. Wang, D. J. Seward, L. Li, J. L. Boyer, and N. Ballatori. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc. Natl. Acad. Sci. USA 98:9431–9436 (2001).
D. J. Seward, A. S. Koh, J. L. Boyer, and N. Ballatori. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J. Biol. Chem. 278:27473–27482 (2003).
P. A. Dawson, M. Hubbert, J. Haywood, A. L. Craddock, N. Zerangue, W. V. Christian, and N. Ballatori. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280:6960–6968 (2005).
R. G. Tirona, and R. B. Kim. Nuclear receptors and drug disposition gene regulation. J. Pharm. Sci. 94:1169–1186 (2005).
M. Makishima. Nuclear receptors as targets for drug development: regulation of cholesterol and bile acid metabolism by nuclear receptors. J. Pharmacol. Sci. 97:177–183 (2005).
J. L. Boyer, M. Trauner, A. Mennone, C. J. Soroka, S. Y. Cai, T. Moustafa, G. Zollner, J. Y. Lee, and N. Ballatori. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol.: Gastrointest. Liver Physiol. 290:G1124–G1130 (2006).
T. Frankenberg, A. Rao, F. Chen, J. Haywood, B. L. Shneider, and P. A. Dawson. Regulation of the mouse organic solute transporter {alpha}-beta, Ost{alpha}-Ostbeta, by bile acids. Am. J. Physiol.: Gastrointest. Liver Physiol. 290:G912–G922 (2006).
J. F. Landrier, J. J. Eloranta, S. R. Vavricka, and G. A. Kullak-Ublick. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiol.: Gastrointest. Liver Physiol. 290:G476–G485 (2006).
H. Lee, Y. Zhang, F. Y. Lee, S. F. Nelson, F. J. Gonzalez, and P. A. Edwards. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J. Lipid Res. 47:201–214 (2006).
G. Zollner, M. Wagner, T. Moustafa, P. Fickert, D. Silbert, J. Gumhold, A. Fuchsbichler, E. Halilbasic, H. Denk, H. U. Marschall, and M. Trauner. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am. J. Physiol.: Gastrointest. Liver Physiol. 290:G923–G932 (2006).
M. Crestani, E. De Fabiani, D. Caruso, N. Mitro, F. Gilardi, A. B. Vigil Chacon, R. Patelli, C. Godio, and G. Galli. LXR (liver X receptor) and HNF-4 (hepatocyte nuclear factor-4): key regulators in reverse cholesterol transport. Biochem. Soc. Trans. 32:92–96 (2004).
D. Jung, B. Hagenbuch, M. Fried, P. J. Meier, and G. A. Kullak-Ublick. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am. J. Physiol.: Gastrointest. Liver Physiol. 286:G752–G761 (2004).
J. Grober, I. Zaghini, H. Fujii, S. A. Jones, S. A. Kliewer, T. M. Willson, T. Ono, and P. Besnard. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 274:29749–29754 (1999).
J. Cui, L. Huang, A. Zhao, J. L. Lew, J. Yu, S. Sahoo, P. T. Meinke, I. Royo, F. Pelaez, and S. D. Wright. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 278:10214–10220 (2003).
H. Nozawa. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem. Biophys. Res. Commun. 336:754–761 (2005).
F. Chen, L. Ma, P. A. Dawson, C. J. Sinal, E. Sehayek, F. J. Gonzalez, J. Breslow, M. Ananthanarayanan, and B. L. Shneider. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 278:19909–19916 (2003).
C. J. Sinal, M. Tohkin, M. Miyata, J. M. Ward, G. Lambert, and F. J. Gonzalez. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 (2000).
C. Thomas, J. F. Landrier, D. Gaillard, J. Grober, M. C. Monnot, A. Athias, and P. Besnard. Cholesterol dependent downregulation of mouse and human apical sodium dependent bile acid transporter (ASBT) gene expression: molecular mechanism and physiological consequences. Gut 55:1321–1331 (2006).
J. F. Landrier, J. Grober, J. Demydchuk, and P. Besnard. FXRE can function as an LXRE in the promoter of human ileal bile acid-binding protein (I–BABP) gene. FEBS Lett. 553:299–303 (2003).
P. A. Mak, H. R. Kast-Woelbern, A. M. Anisfeld, and P. A. Edwards. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J. Lipid Res. 43:2037–2041 (2002).
T. Li, and J. Y. Chiang. Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression. Drug Metab. Dispos. 34:756–764 (2006).
Acknowledgment
This work was supported by grants from The Japanese Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Okuwaki and T. Takada contributed equally to this work.
Rights and permissions
About this article
Cite this article
Okuwaki, M., Takada, T., Iwayanagi, Y. et al. LXR Alpha Transactivates Mouse Organic Solute Transporter Alpha and Beta via IR-1 Elements Shared with FXR. Pharm Res 24, 390–398 (2007). https://doi.org/10.1007/s11095-006-9163-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9163-6