Abstract
Purpose
Methotrexate (MTX) causes dose-limiting gastrointestinal toxicity due to exposure of intestinal tissues, and is a substrate of the multidrug resistance-associated protein (MRP) 1. Here we examine the involvement of MRP1, which is reported to be highly expressed in the proliferative crypt compartment of the small intestine, in the gastrointestinal toxicity of MTX.
Methods
MTX was intraperitonealy administered to mrp1 gene knockout (mrp1 (−/−)) and wild-type (mrp1 (+/+)) mice. Body weight, food and water intake were monitored, intestinal histological studies and pharmacokinetics of MTX were examined.
Results
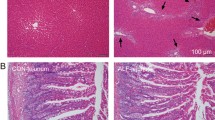
mrp1 (−/−) mice more severely decreased body weight, food and water intake than mrp1 (+/+) mice. Almost complete loss of villi throughout the small intestine in mrp1 (−/−) mice was observed, whereas the damage was only partial in mrp1 (+/+) mice. Plasma concentration and biliary excretion profiles of MTX were similar in mrp1 (−/−) and mrp1 (+/+) mice, though accumulation of MTX in immature proliferative cells isolated from mrp1 (−/−) mice was much higher compared to mrp1 (+/+) mice. Immunostaining revealed localization of Mrp1 in plasma membrane of the intestinal crypt compartment in mrp1 (+/+) mice, but not in mrp1 (−/−) mice.
Conclusion
Mrp1 determines the exposure of proliferative cells in the small intestine to MTX, followed by gastrointestinal toxicity.




Similar content being viewed by others
References
K. Welte, M. A. Bonilla, A. P. Gillio, T. C. Boone, G. K. Potter, J. L. Gabrilove, M. A. Moore, R. J. O'Reilly, and L. M. Souza. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J. Exp. Med. 165:941–948 (1987) doi:10.1084/jem.165.4.941.
S. E. Steinberg, C. L. Campbell, W. A. Bleyer, and R. S. Hillman. Enterohepatic circulation of methotrexate in rats in vivo. Cancer Res. 42:1279–1282 (1982).
D. Griffin, and H. M. Said. The enterohepatic circulation of methotrexate in vivo: inhibition by bile salt. Cancer Chemother. Pharmacol. 19:40–41 (1987) doi:10.1007/BF00296253.
M. Masuda, Y. I’izuka, M. Yamazaki, R. Nishigaki, Y. Kato, K. Ni'inuma, H. Suzuki, and Y. Sugiyama. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res. 57:3506–3510 (1997).
P. Breedveld, N. Zelcer, D. Pluim, O. Sönmezer, M. M. Tibben, J. H. Beijnen, A. H Schinkel, O. van Tellingen, P. Borst, and J. H. Schellens. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 64:5804–5811 (2004) doi:10.1158/0008-5472.CAN-03-4062.
M. Takeda, S. Khamdang, S. Narikawa, H. Kimura, M. Hosoyamada, S. H. Cha, T. Sekine, and H. Endou. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J. Pharmacol. Exp. Ther. 302:666–671 (2002) doi:10.1124/jpet.102.034330.
K. Miyamoto, T. Shiraga, K. Morita, H. Yamamoto, H. Haga, Y. Taketani, I. Tamai, Y. Sai, A. Tsuji, and E. Takeda. Sequence, tissue distribution and developmental change in rat intestinal oligopeptide transporter. Biochim. Biophys. Acta. 1305:34–38 (1996).
I. Tamai, T. Nakanishi, K. Hayashi, T. Terao, Y. Sai, T. Shiraga, K. Miyamoto, E. Takeda, H. Higashida, and A. Tsuji. The predominant contribution of oligopeptide transporter PepT1 to intestinal absorption of ß-lactam antibiotics in the rat small intestine. J. Pharm. Pharmacol. 49:796–801 (1997).
Y. Wang, R. Zhao, R. G. Russell, and I. D. Goldman. Localization of murine reduced folate carrier as assessed by immuhistochemical analysis. Biochim. Biophys. Acta. 1513:49–54 (2001) doi:10.1016/S0005-2736(01)00340-6.
M. Shayeghi, G. O. Latunde-Dada, J. S. Oakhill, A. H. Laftah, K. Takeuchi, N. Halliday, Y. Khan, A. Warley, F. E. McCann, R. C. Hider, D. M. Frazer, G. J. Anderson, C. D. Vulpe, R. J. Simpson, and A. T. McKie. Identification of an intestinal heme transporter. Cell. 122:789–801 (2005) doi:10.1016/j.cell.2005.06.025.
Y. Wang, A. Rajgopal, I. D. Goldman, and R. Zhao. Preservation of folate transport activity with a low-pH optimum in rat IEC-6 intestinal epithelial cell lines that lack reduced folate carrier function. Am. J. Physiol. 288:C65–C71 (2005) doi:10.1152/ajpcell.00533.2004.
A. Qiu, M. Jansen, A. Sakaris, S. H. Min, S. Chattopadhyay, E. Tsai, C. Sandoval, R. Zhao, M. H. Akabas, and I. D. Goldman. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 127:917–928 (2006) doi:10.1016/j.cell.2006.09.041.
T. Terao, E. Hisanaga, Y. Sai, I. Tamai, and A. Tsuji. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol. 48:1083–1089 (1996).
K. Naruhashi, I. Tamai, N. Inoue, H. Muraoka, Y. Sai, N. Suzuki, and A. Tsuji. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob. Agents Chemother. 46:344–349 (2002) doi:10.1128/AAC.46.2.344-349.2002.
D. Rost, S. Mahner, Y. Sugiyama, and W. Stremmel. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am. J. Physiol. 28:G720–G726 (2002).
H. Zeng, Z. S. Chen, M. G. Belinsky, P. A. Rea, and G. D. Kruh. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res. 61:7225–7232 (2001).
K. C. Peng, F. Cluzeaud, M. Bens, J. P. Van Huyen, M. A. Wioland, R. Lacave, and A. Vandewalle. Tissue and cell distribution of the multidrug resistance associated protein (MRP) in mouse intestine and kidney. J. Histochem. Cytochem. 47:757–767 (1999).
J. Wijnholds, R. Evers, M. R. van Leusden, C. A. Mol, G. J. Zaman, U. Mayer, J. H. Beijnen, M. van der Valk, P. Krimpenfort, and P. Borst. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 3:1275–1279 (1997) doi:10.1038/nm1197-1275.
A. Kurita, S. Kado, N. Kaneda, M. Onoue, S. Hashimoto, and T. Yokokura. Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother. Pharmacol. 46:211–220 (2000) doi:10.1007/s002800000151.
O. C. Trifan, W. F. Durham, V. S. Salazar, J. Horton, B. D. Levine, B. S. Zweifel, T. W. Davis, and J. L. Masferrer. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 62:5778–5784 (2002).
F. M. Sirotnak, D. M. Moccio, and C. H. Yang. Similar characteristics of folate analogue transport in vitro in contrast to varying dihydrofolate reductase levels in epithelial cells at different stages of maturation in mouse small intestine. Cancer Res. 44:5204–5211 (1984).
M. M. Weiser. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J. Biol. Chem. 248:2536–2541 (1973).
K. Ueda, Y. Kato, K. Komatsu, and Y. Sugiyama. Inhibition of biliary excretion of methotrexate by probenecid in rats: quantitative prediction of interaction from in vitro data. J. Pharmacol. Exp. Ther. 297:1036–1043 (2001).
E. S. Henderson, R. H. Adamson, C. Denham, and V. T. Oliverio. The metabolic fate of tritiated methotrexate 1. Absorption, excretion, and distribution in mice, rats, dogs and monkeys. Cancer Res. 25:1008–1017 (1965).
A. Lorico, G. Rappa, R. A. Finch, D. Yang, R. A. Flavell, and A. C. Sartorelli. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 57:5238–5242 (1997).
L. J. Bain, and R. A. Feldman. Altered expression of sulfotransferases, glucuronosyltransferases and mrp transporters in FVB/mrp1-/- mice. Xenobiotica. 33:1173–1183 (2003) doi:10.1080/00498250310001609138.
H. Cheng, and C. P. Leblond. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141:537–561 (1974) doi:10.1002/aja.1001410407.
C. A. Loehry, D. N. Croft, A. K. Singh, and B. Creamer. Cell turnover in the rat small intestinal mucosa: an appraisal of cell loss. Gut. 10:13–16 (1969) doi:10.1136/gut.10.1.13.
I. B. Renes, M. Verburg, N. P. Bulsing, S. Ferdinandusse, H. A. Büller, J. Dekker, and A. W. Einerhand. Protection of the Peyer's patch-associated crypt and villus epithelium against methotrexate-induced damage is based on its distinct regulation of proliferation. J. Pathol. 198:60–68 (2002) doi:10.1002/path.1183.
Y. Miyazono, F. Gao, and T. Horie. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand. J. Gastroenterol. 39:1119–1127 (2004) doi:10.1080/00365520410003605.
B. A. de Koning, J. M. van Dieren, D. J. Lindenbergh-Kortleve, M. van der Sluis, T. Matsumoto, K. Yamaguchi, A. W. Einerhand, J. N. Samsom, R. Pieters, and E. E. Nieuwenhuis. Contributions of mucosal immune cells to methotrexate-induced mucositis. 1. Int. Immunol. 18:941–949 (2006) doi:10.1093/intimm/dxl030.
B. A. de Koning, M. Sluis, D. J. Lindenbergh-Kortleve, A. Velcich, R. Pieters, H. A. Büller, A. W. Einerhand, and I. B. Renes. Methotrexate-induced mucositis in mucin 2-deficient mice. J. Cell. Physiol. 210:144–152 (2007) doi:10.1002/jcp.20822.
J. Wijnholds, G. L. Scheffer, M. van der Valk, P. van der Valk, J. H. Beijnen, R. J. Scheper, and P. Borst. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J. Exp. Med. 188:797–808 (1998) doi:10.1084/jem.188.5.797.
M. G. Belinsky, P. Guo, K. Lee, F. Zhou, E. Kotova, A. Grinberg, H. Westphal, I. Shchaveleva, A. Klein-Szanto, J. M. Gallo, and G. D. Kruh. Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer Res. 67:262–268 (2007) doi:10.1158/0008-5472.CAN-06-2680.
Y. Mochida, K. Taguchi, S. Taniguchi, M. Tsuneyoshi, H. Kuwano, T. Tsuzuki, M. Kuwano, and M. Wada. The role of P-glycoprotein in intestinal tumorigenesis: disruption of mdr1a suppresses polyp formation in Apc(Min/+) mice. Carcinogenesis. 24:1219–1224 (2003) doi:10.1093/carcin/bgg073.
H. Wang, X. Wu, K. Hudkins, A. Mikheev, H. Zhang, A. Gupta, J. D. Unadkat, and Q. Mao. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am. J. Physiol. Endocrinol. Metab. 291:E1295–E1304 (2006) doi:10.1152/ajpendo.00193.2006.
H. Bleiberg. CPT-11 in gastrointestinal cancer. Eur. J. Cancer. 35:371–379 (1999) doi:10.1016/S0959-8049(98)00423-7.
J. T. Hartmann, and H. P. Lipp. Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II—mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf. 29:209–230 (2006) doi:10.2165/00002018-200629030-00005.
Z. S. Chen, T. Furukawa, T. Sumizawa, K. Ono, K. Ueda, K. Seto, and S. I. Akiyama. ATP-Dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol. Pharmacol. 55:921–928 (1999).
Acknowledgement
We thank Ms. Lica Ishida and Mr. Syuichi Yamazaki for technical assistance. This work was supported in part by a Grant-in-Aid for Scientific Research provided by the Ministry of Education, Science and Culture of Japan and funds from the Kanehara Grant Program 2004 of the Ichiro Kanehara Foundation (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, S., Ito, K., Kato, Y. et al. Involvement of Multidrug Resistance-Associated Protein 1 in Intestinal Toxicity of Methotrexate. Pharm Res 26, 1467–1476 (2009). https://doi.org/10.1007/s11095-009-9858-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9858-6