ABSTRACT
Purpose
To develop a semi-mechanistic model linking in vitro to in vivo drug release.
Methods
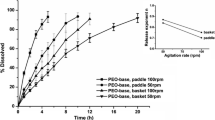
A nonlinear mixed-effects model describing the in vitro drug release for 6 hydrophilic matrix based modified release formulations across different experimental conditions (pH, rotation speed and ionic strength) was developed. It was applied to in vivo observations of drug release and tablet gastro intestinal (GI) position assessed with magnetic marker monitoring (MMM). By combining the MMM observations with literature information on pH and ionic strength along the GI tract, the mechanical stress in different parts of the GI tract could be estimated in units equivalent to rotation speed in the in vitro USP 2 apparatus.
Results
The mechanical stress in the upper and lower stomach was estimated to 94 and 134 rpm, respectively. For the small intestine and colon the estimates of mechanical stress was 93 and 38 rpm. Predictions of in vivo drug release including between subject/tablet variability was made for other newly developed formulations based on the drug release model and a model describing tablet GI transit.
Conclusion
The paper outlines a modeling approach for predicting in vivo behavior from standard in vitro experiments and support formulation development and quality control.






Similar content being viewed by others
REFERENCES
Tong C, Lozano R, Mao Y, Mirza T, Löbenberg R, Nickerson B, et al. The value of in Vitro dissolution in drug development: a position paper from the AAPS in Vitro release and dissolution focus group. Pharm Technol. 2009;33(4):52–64.
Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1 SPEC. ISS):12–21.
Siewert M. FIP guidelines for dissolution testing of solid oral products. Joint report of the section for official laboratories and medicines control services and the section of industrial pharmacists of the F.I.P. Drugs Made in Germany. 1997;40(4):123–9
Jorgensen ED, Bhagwat D. Development of dissolution tests for oral extended-release products. Pharm Sci Tech Today. 1998;1(3):128–35.
Garbacz G, Golke B, Wedemeyer RS, Axell M, Söderlind E, Abrahamsson B, et al. Comparison of dissolution profiles obtained from nifedipine extended release once a day products using different dissolution test apparatuses. Eur J Pharm Sci. 2009;38(2):147–55.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting In vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: An update. Pharm Res. 2008;25(7):1663–76.
Jantratid E, De Maio V, Ronda E, Mattavelli V, Vertzoni M, Dressman JB. Application of biorelevant dissolution tests to the prediction of in vivo performance of diclofenac sodium from an oral modified-release pellet dosage form. Eur J Pharm Sci. 2009;37(3–4):434–41.
Heigoldt U, Sommer F, Daniels R, Wagner KG. Predicting in vivo absorption behavior of oral modified release dosage forms containing pH-dependent poorly soluble drugs using a novel pH-adjusted biphasic in vitro dissolution test. Eur J Pharm Biopharm. 2010;76(1):105–11.
Abrahamsson B, Pal A, Sjöberg M, Carlsson M, Laurell E, Brasseur JG. A novel in Vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharm Res. 2005;22(8):1215–26.
Garbacz G, Wedemeyer RS, Nagel S, Giessmann T, Monnikes H, Wilson CG, et al. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur J Pharm Biopharm. 2008;70(2):421–8.
Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, et al. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–14.
Shono Y, Jantratid E, Kesisoglou F, Reppas C, Dressman JB. Forecasting in vivo oral absorption and food effect of micronized and nanosized aprepitant formulations in humans. Eur J Pharm Biopharm. 2010;76(1):95–104.
Willmann S, Thelen K, Becker C, Dressman JB, Lippert J. Mechanism-based prediction of particle size-dependent dissolution and absorption: Cilostazol pharmacokinetics in dogs. Eur J Pharm Biopharm. 2010;76(1):83–94.
Soto E, Haertter S, Koenen-Bergmann M, Staab A, Trocóniz IF. Population in vitro-in vivo correlation model for pramipexole slow-release oral formulations. Pharm Res. 2010;27(2):340–9.
Lukacova V, Woltosz WS, Bolger MB. Prediction of modified release pharmacokinetics and pharmacodynamics from in vitro, immediate release, and intravenous data. AAPS J. 2009;11(2):323–34.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–37.
Tubic-Grozdanis M, Bolger MB, Langguth P. Application of gastrointestinal simulation for extensions for biowaivers of highly permeable compounds. AAPS J. 2008;10(1):213–26.
Willmann S, Edginton AN, Kleine-Besten M, Jantratid E, Thelen K, Dressman JB. Whole-body physiologically based pharmacokinetic population modelling of oral drug administration: inter-individual variability of cimetidine absorption. J Pharm Pharmacol. 2009;61(7):891–9.
Weitschies W, Blume H, Mönnikes H. Magnetic Marker Monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm. 2009;74(1):93–101.
Weitschies W, Hartmann V, Grutzmann R, Breitkreutz J. Determination of the disintegration behavior of magnetically marked tablets. Eur J Pharm Biopharm. 2001;52(2):221–6.
Bergstrand M, Söderlind E, Weitschies W, Karlsson MO. Mechanistic modeling of a magnetic marker monitoring study linking gastrointestinal tablet transit, in vivo drug release, and pharmacokinetics. Clin Pharmacol Ther. 2009 Jul;86(1):77–83.
Johansson S, Cullberg M, Eriksson UG, Elg M, Dunér K, Jensen E, et al. Single-dose pharmacokinetics, pharmacodynamics and safety of AZD0837, a novel oral direct thrombin inhibitor, in young healthy male subjects. Int J Clin Pharm Ther. 2011;49(4):258–67.
Lip GYH, Lane DA. Does warfarin for stroke thromboprophylaxis protect against MI in atrial fibrillation patients? Am J Med. 2010;123(9):785–9.
Weitschies W, Kosch O, Mönnikes H, Trahms L. Magnetic Marker Monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv Drug Deliv Rev. 2005;57(8):1210–22.
Beal SL, Sheiner LB, Boeckmann AJ. NONMEM User’s Guides. Ellicot City: MD: Icon Development Solutions; 1989–2006.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Bergstrand M, Karlsson MO. Handling Data Below the Limit of Quantification in Mixed Effect Models. AAPS J. 2009;11(2):371–80.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Meth Prog Biomed. 2005;79(3):241–57.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Meth Prog Biomed. 2004;75(2):85–94.
Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Prog Biomed. 1999;58(1):51–64.
Harling K, Ueckert S, Hooker AC, Jonsson EN, Karlsson MO. Xpose and Perl speaks NONMEM (PsN). PAGE 19 (2010) Abstr 1842 [wwwpage-meetingorg/?abstract=1842].
R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2009 [2009-12-30]; Available from: http://www.R-project.org.
Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24(12):979–81.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Euro J Pharm Sci. 2001;13(2):123–33.
Hopfenberg HB. Controlled release from erodible slabs, cylinders and spheres. Controlled Release Polymeric Formulations. 1976;33:26–32.
Baker RW, Lonsdale HK. Controlled release: mechanisms and rates. Controlled Release of Biologically Active Agents. 1974:15–71.
Bolourchian N, Dadashzadeh S. pH-independent release of propranolol hydrochloride from HPMC-based matrices using organic acids. Daru. 2008;16(3):136–42.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: Problems and solutions. AAPS J. 2009;11(3):558–69.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007 Jul;82(1):17–20.
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988 Aug;29(8):1035–41.
Simonian HP, Vo L, Doma S, Fisher RS, Parkman HP. Regional postprandial differences in pH within the stomach and gastroesophageal junction. Dig Dis Sci. 2005 Dec;50(12):2276–85.
Lindahl A, Ungell AL, Knutson L, Lennernas H. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm Res. 1997 Apr;14(4):497–502.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011; in press.
Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27(8):886–92.
Yuen KH. The transit of dosage forms through the small intestine. Int J Pharm. 2010;395(1–2):9–16.
Guidance for industry: Extended release oral dosage forms: Development, evaluation, and application of in vitro/in vivo correlations. Food and Drug Administration. 1997;1–27.
Garbacz G, Klein S, Weitschies W. A biorelevant dissolution stress test device background and experiences. Expert Opinion Drug Deliv. 2010;7(11):1251–61.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 91 kb)
Rights and permissions
About this article
Cite this article
Bergstrand, M., Söderlind, E., Eriksson, U.G. et al. A Semi-mechanistic Modeling Strategy to Link In Vitro and In Vivo Drug Release for Modified Release Formulations. Pharm Res 29, 695–706 (2012). https://doi.org/10.1007/s11095-011-0594-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0594-3