Abstract
Purpose
To develop a physiologically based pharmacokinetic model in adults and children for clobazam, its active metabolite norclobazam and stiripentol and to account for significant clinical interaction that has been reported when clobazam and stiripentol are co-administered.
Methods
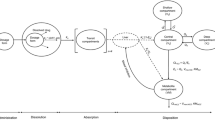
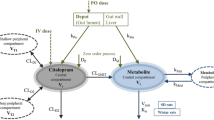
A PBPK model with ten compartments was developed. An in vitro-in vivo extrapolation technique was used to scale clearance in children for clobazam and norclobazam and clearance parameters for stiripentol were obtained from fitting. Other drug and system parameters were obtained from the literature.
Results
The tissue/blood partition coefficients adequately predict observed volume of distribution for clobazam and stiripentol. In a clinical study in children where clobazam was administered alone and co-administered with stiripentol, the predicted and observed minimum concentration at steady state (mean and 95% confidence interval) during clobazam monotherapy were 0.19 (0.05–0.49 mg/L) and 0.20 (0.17–0.23 mg/L), respectively, and predicted and observed norclobazam concentrations were 0.49 (0.16–1.38 mg/L) and 0.95 (0.91–0.99 mg/L), respectively. From an interaction study with stiripentol the predicted stiripentol concentration was 10.12 (2.51–39.36 mg/L) and the observed concentration was 10.0 (8.3–11.7 mg/L); the predicted clobazam concentration was 0.29 (0.07–1.05 mg/L) and the observed concentration was 0.31 (0.24–0.38 mg/L); and the predicted norclobazam concentration was 2.30 (0.45–5.53 mg/L) and the observed concentration was 4.32 (3.77–4.87 mg/L).
Conclusions
The PBPK model adequately described observed data and the extent of interaction between clobazam/norclobazam and stiripentol.












Similar content being viewed by others
References
Hurst DL. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia. 1990;31:397–400.
Morse RP. Dravet syndrome: inroads into understanding epileptic encephalopathies. J Pediatr. 2011;158:354–9.
Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia. 2011;52 Suppl 2:72–5.
Plosker GL. Stiripentol: in severe myoclonic epilepsy of infancy (dravet syndrome). CNS Drugs. 2012;26:993–1001.
Chiron C, Marchand MC, Tran A, Rey E, Athis P, Vincent J, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356:1638–42.
Greenblatt DJ, Divoll M, Abernethy DR, Ochs HR, Shader RI. Clinical pharmacokinetics of the newer benzodiazepines. Clin Pharmacokinet. 1983;8:233–52.
Giraud C, Tran A, Rey E, Vincent J, Treluyer JM, Pons G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004;32:1279–86.
Greenblatt DJ, Divoll M, Puri SK, Ho I, Zinny MA, Shader RI. Clobazam kinetics in the elderly. Br J Clin Pharmacol. 1981;12:631–6.
Chiron C. Stiripentol Neurother. 2007;4:123–5.
Moreland TA, Astoin J, Lepage F, Tombret F, Levy RH, Baillie TA. The metabolic fate of stiripentol in man. Drug Metab Dispos. 1986;14:654–62.
Chhun S, Peigne S, Rey E, Pons G, Jullien V. Population pharmacokinetic study in healthy volunteers treated with 3 single doses of stiripentol (500, 1,000, 2,000 mg). Fundam Clin Pharmacol. 2009;23:24–5.
Levy RH, Lin HS, Blehaut HM, Tor JA. Pharmacokinetics of stiripentol in normal man: evidence of nonlinearity. J Clin Pharmacol. 1983;23:523–33.
Giraud C, Treluyer JM, Rey E, Chiron C, Vincent J, Pons G, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34:608–11.
Tran A, Rey E, Pons G, Rousseau M, d’Athis P, Olive G, et al. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: in vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin Pharmacol Ther. 1997;62:490–504.
Levy RH, Loiseau P, Guyot M, Blehaut HM, Tor J, Moreland TA. Stiripentol kinetics in epilepsy: nonlinearity and interactions. Clin Pharmacol Ther. 1984;36:661–9.
Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values: ICRP Publication 89. Ann ICRP. 2002;32:1–277.
Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94:1259–76.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11:225–37.
Levy RH, Loiseau P, Guyot M, Blehaut HM, Tor J, Moreland TA. Michaelis-Menten kinetics of stiripentol in normal humans. Epilepsia. 1984;25:486–91.
Rey E, Jullien V, D’Athis P, Vincent J, Pons G. Is pharmacokinetics of stiripentol linear? Clin Pharmacol Ther. 2005;77:P64–4.
Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s guides. (1989–2009). Ellicott City: Icon Development Solutions; 2009.
Menke G, Pfister P, Sauerwein S, Rietbrock I, Woodcock BG, Rietbrock N. Age-dependence and free fatty acid modulation of binding kinetics at the benzodiazepine binding site of serum albumin in neonates and adults determined using fast reaction methods. Br J Clin Pharmacol. 1987;23:439–45.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4:E4.
Salem F, Johnson TN, Barter ZE, Leeder JS, Rostami-Hodjegan A. Age related changes in fractional elimination pathways for drugs: assessing the impact of variable ontogeny on metabolic drug-drug interactions. J Clin Pharmacol. 2013;53:857–65.
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–9.
GetData Graph Digitizer, http://getdata-graph-digitizer.com/2013.
MATLAB 7.14.0.739. Natick, Massachusetts: The MathWorks Inc. R 2012a.
Tedeschi G, Riva R, Baruzzi A. Clobazam plasma-concentrations - pharmacokinetic study in healthy-volunteers and data in epileptic patients. Br J Clin Pharmacol. 1981;11:619–22.
Ochs HR, Greenblatt DJ, Luttkenhorst M, Verburg-Ochs B. Single and multiple dose kinetics of clobazam, and clinical effects during multiple dosage. Eur J Clin Pharmacol. 1984;26:499–503.
Greenblatt DJ. Electron-capture GLC determination of clobazam and desmethylclobazam in plasma. J Pharm Sci. 1980;69:1351–2.
Divoll M, Greenblatt DJ, Ciraulo DA, Puri SK, Ho I, Shader RI. Clobazam kinetics: intrasubject variability and effect of food on adsorption. J Clin Pharmacol. 1982;22:69–73.
Bun H, Coassolo P, Gouezo F, Serradimigni A, Cano JP. Time-dependence of clobazam and N-demethylclobazam kinetics in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1986;24:287–93.
Greenblatt DJ, Divoll M, Puri SK, Ho I, Zinny MA, Shader RI. Reduced single-dose clearance of clobazam in elderly men predicts increased multiple-dose accumulation. Clin Pharmacokinet. 1983;8:83–94.
Pullar T, Edwards D, Haigh JR, Peaker S, Feely MP. The effect of cimetidine on the single dose pharmacokinetics of oral clobazam and N-desmethylclobazam. Br J Clin Pharmacol. 1987;23:317–21.
Jawad S, Richens A, Oxley J. Single dose pharmacokinetic study of clobazam in normal volunteers and epileptic patients. Br J Clin Pharmacol. 1984;18:873–7.
Vallner JJ, Kotzan JA, Stewart JT, Honigberg IL, Needham TE, Brown WJ. Plasma levels of clobazam after 10-, 20-, and 40-mg tablet doses in healthy subjects. J Clin Pharmacol. 1980;20:444–51.
May TW, Boor R, Mayer T, Jurgens U, Rambeck B, Holert N, et al. Concentrations of stiripentol in children and adults with epilepsy: the influence of dose, age, and comedication. Ther Drug Monit. 2012;34:390–7.
Tran A, Vauzelle-Kervroedan F, Rey E, Pous G, d’Athis P, Chiron C, et al. Effect of stiripentol on carbamazepine plasma concentration and metabolism in epileptic children. Eur J Clin Pharmacol. 1996;50:497–500.
Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P, et al. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40:1618–26.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15:455–64.
Pullar T, Haigh JR, Peaker S, Feely MP. Pharmacokinetics of N-desmethylclobazam in healthy volunteers and patients with epilepsy. Br J Clin Pharmacol. 1987;24:793–7.
Vallner JJ, Needham TE, Jun HW, Brown WJ, Stewart JT, Kotzan JA, et al. Plasma levels of clobazam after three oral dosage forms in health subjects. J Clin Pharmacol. 1978;18:319–24.
Rupp W, Badian M, Christ O, Hajdu P, Kulkarni RD, Taeuber K, et al. Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol. 1979;7 Suppl 1:51S–7S.
L. Inc. Onfi (clobazam) Tablets for oral use: prescribing information 2011.
Volz M, Christ O, Kellner HM, Kuch H, Fehlhaber HW, Gantz D, et al. Kinetics and metabolism of clobazam in animals and man. Br J Clin Pharmacol. 1979;7 Suppl 1:41S–50S.
Acknowledgments and Disclosures
This work performed as part of Child-Rare-Euro-Simulation project. CRESim was funded by the ERA-NET PRIOMEDCHILD Joint Call in 2010. Members of the CRESim Project Group: Leon Aarons; Agathe Bajard; Clément Ballot; Yves Bertrand; Frank Bretz; Daan Caudri; Charlotte Castellan; Sylvie Chabaud; Catherine Cornu; Frank Dufour; Nathalie Eymard; Roland Fisch; Renzo Guerrini; Vincent Jullien; Behrouz Kassaï; Patrice Nony; Kayode Ogungbenro; David Pérol; Gérard Pons; Harm Tiddens; Anna Rosati. Members of the Epi-CRESim Project Group: Corinne Alberti; Catherine Chiron; Catherine Cornu, Polina Kurbatova; Rima Nabbout.
The authors also acknowledge helpful discussions with Aleksandra Galetin, Michael Gertz, Eleanor Howgate, Henry Pertinez, Nikolaos Tsamandouras, Adam Darwich and members of Centre for Applied Pharmacokinetic Research, Manchester Pharmacy School.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ogungbenro, K., Aarons, L. & and the CRESim & Epi-CRESim Project Groups. A Physiologically Based Pharmacokinetic Model for Clobazam and Stiripentol in Adults and Children. Pharm Res 32, 144–157 (2015). https://doi.org/10.1007/s11095-014-1451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1451-y