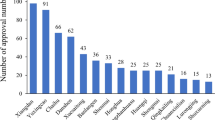
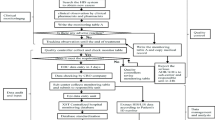
Abstract
Objective This study aimed to evaluate the safety for an injection with a mixture of extracts from Herba Artemisiae annuae, Fructus Gardeniae and Flos Lonicerae and to determine the risk factors that may affect its adverse drug reactions. Methods A drug-oriented prospective observational study was performed. Physicians filled in clinical observation forms with detailed information of the patients including general information, drug information, therapeutic effects and adverse drug events. The adverse drug reaction factors were analyzed by both mono-factor and multiple-factor logistic regression methods. Results From April to July 2007, we collected 12,427 observation forms from 46 hospitals in Jiangsu Province of China. Among the 11,707 observation forms we analyzed, 8,074 patients were children younger than 14 years old (69%). Among 51 reported adverse drug events, 45 cases were adverse drug reactions. The total adverse drug reaction incidence of the injection was 0.38%. While most adverse drug reactions were previously known (e.g., rash, pruritus, vomiting and diarrhea), we observed three new ADR symptoms: shiver, phlebitis and anhelation. All the adverse drug reactions were controlled very well through the follow-up therapy, and none of them was life threatening. The mono-factor analysis showed that adverse drug reactions of the injection were significantly correlated with total medication dose (P = 0.0049) and combination medication (P = 0.0143), especially with antimicrobial drugs (P = 0.0079) and macrolides (P = 0.0017). The multiple factor analysis confirmed these results: medication dosage and combination medication had a crucial impact on adverse drug reactions of the injection; the risk was increased by 24.8% (the estimated value of relative risk was 1.248, 95% confidence interval: 1.054–1.479) and 89% (1.890, 1.001–3.566), respectively. Conclusion The total adverse drug reaction incidence of the injection was 0.38% and lower than we expected. Moreover, we observed three new adverse drug reactions, none of which was severe.
Similar content being viewed by others
Notes
The Chinese brand name of the injection with a mixture of extracts was Reduning injection®
References
Wang JG. Intensive monitoring of ADRs. Chin J Pharmacoepidemiol. 1997;6(1):6–11.
Xiao W, Ling Y, Bi YA, et al. GC/MS fingerprint of reduning injection. Chin J Nat Med. 2007;5(2):127–9.
Feng GZ, Zhou F, Huang M. Inhibitory effects of reduning injection on influenza virus FM1 in vitro. Chin J N Drug Clin Rem. 2007;26(9):663–7.
Feng GZ, Zhou F, Huang M. Anti-respiratory syncytial virus (RSV, Long strain) effects of reduning injection in vitro. Acta Univ Med Nanjing. 2007;27(9):1009–12 (Nature science).
Feng GZ, Zhou F, Huang M. Inhibitory effects of reduning injection on adenovirus type-3 in vitro. Chin J N Drug Clin Rem. 2007;26(8):573–7.
Chen HZ. Practical of internal medicine. 12nd ed. Beijing: The People Hygiene Publishing Company, 2005. ISBN 7-117-06595-8.
Song Z, Xu HP, Lu F, et al. Therapeutic effect observation of reduning injection on acute trache- bronchitis affection of exogenous wind-heat. Hubei TCM J. 2007;29(4):82.
Cai XP, Ma YF, He ZB, et al. Clinical observation of reduning injection on affection of exogenous wind-heat. Pract J Card Cereb Pneumal Vasc Dis. 2006;14(5):182–3.
Xu HP, Tang ZT, Lu F, et al. Clinical observation of reduning injection on affection of exogenous wind-heat of children. J Emerg Tradit Chin Med. 2008;17(9):1812–5.
Ding YF. Allergy induced by traditional Chinese medicine and influential factor. Cent China Med J. 2007;31(4):244–6.
Jankel CA, Speedie SM. Detecting drug interactions: a review of the literature. Ann Pharmacother. 1990;24(10):982–9.
Guo YG, Zhang JJ, Si DY, et al. Effects of the active components of Chinese herbs on CYP related genes expression in hepG2 Cells. Tradit Chin Drug Res Clin Pharmacol. 2008;19(4):243–6.
Asimus S, Elsherbiny D, Hai TN, et al. Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in health subjects. Fundam Clin Pharmacol. 2007;21(3):307–16. doi:10.1111/j.1472-8206.2007.00471.x.
Sotaniemi EA, Pelkonen O, Arranto AJ, et al. Diabetes and elimination of antipyrine in man: an analysis of 298 patients classified by type of diabetes, ages, sex, duration of disease and liver involvement. Pharmacol Toxicol. 2002;90(3):155–60. doi:10.1034/j.1600-0773.2002.900308.x.
Kotlyar M, Carson SW. Effectds of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999;37(1):8–19.
Acknowledgements
We would like to thank all the physicians who participated in our study.
Funding
This study was partly supported by Jiangsu Kanion Pharmaceutical Co., Ltd, China.
Conflict of interest
The company that funded the study had no influence on any decisions in relation to study design or publication. There are no other (potential) conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H.M., Wang, Y. & Liu, N.F. Safety of an injection with a mixture of extracts from Herba Artemisiae annuae, Fructus Gardeniae and Flos Lonicerae . Pharm World Sci 31, 458–463 (2009). https://doi.org/10.1007/s11096-009-9297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-009-9297-9