Abstract
Background
Darunavir is a potent protease inhibitor of HIV. To enhance its pharmacokinetic profile, darunavir must be co-administered with ritonavir. There is wide inter-patient variability in darunavir pharmacokinetics among HIV-infected individuals, however. Darunavir is a known substrate for influx transporters, such as the 1A2 and the 1B1 members of the solute carrier organic anion transporter family (SLCO1A2, SLCO1B1), as well as for efflux transporters such as the multi-drug resistance protein 1 (MRP1).
Objective
The aim of this study was to develop a semi-mechanistic population pharmacokinetic model for darunavir and ritonavir administered in HIV-infected adults. The desired model would incorporate patient characteristics and pharmacogenetic data contributing to variability in drug concentrations and also take into account the interaction between the two compounds.
Methods
A population pharmacokinetic analysis was performed with 705 plasma samples from 75 Caucasian individuals receiving darunavir/ritonavir (600/100 mg twice daily) for at least 4 weeks. At least one full pharmacokinetic profile was obtained for each participant, and darunavir and ritonavir concentrations in plasma were determined by high performance liquid chromatography. Genotyping for 148 polymorphisms in genes coding for transporters or metabolizing enzymes was conducted by two methods: MALDI-TOF mass spectrometry and real-time polymerase chain reaction-based allelic discrimination. A population pharmacokinetic model was developed for darunavir and for ritonavir. The effect of single nucleotide polymorphisms on the post hoc individual pharmacokinetic parameters was first explored using graphic methods and regression analysis. Those covariates related to changes in darunavir or ritonavir pharmacokinetic parameters were then further evaluated using non-linear mixed effects modeling (NONMEM version VII).
Results
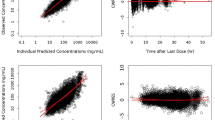
Darunavir and ritonavir pharmacokinetics were best described by a two- and one-compartment model, respectively, both with first-order absorption and elimination. The darunavir peripheral volume of distribution decreased as α1-acid glycoprotein concentrations increased. Darunavir clearance was 12 % lower in patients with SLCO3A1 rs8027174 GT/TT genotypes, while homozygosity for the rs4294800 A allele was associated with 2.5-fold higher central volume of distribution. Body weight influenced ritonavir clearance. Ritonavir inhibited darunavir clearance following a maximum-effect model.
Conclusion
A population pharmacokinetic model to simultaneously describe the pharmacokinetics of darunavir and ritonavir was developed in HIV-infected patients. The model provides better understanding of the interaction between darunavir and ritonavir and suggests an association between SLCO3A1 polymorphisms and darunavir pharmacokinetics. Bayesian estimates of individual darunavir parameters and ritonavir may be useful to predict darunavir exposure.



Similar content being viewed by others
References
Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568):1169–78.
Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23(13):1679–88.
Rittweger M, Arastéh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46(9):739–56.
King JR, Wynn H, Brundage R, et al. Pharmacokinetic enhancement of protease inhibitor therapy. Clin Pharmacokinet. 2004;43(5):291–310.
Autar RS, Boffito M, Hassink E, et al. Interindividual variability of once-daily ritonavir boosted saquinavir pharmacokinetics in Thai and UK patients. J Antimicrob Chemother. 2005;56(5):908–13.
Avihingsanon A, van der Lugt J, Kerr SJ, et al. A low dose of ritonavir-boosted atazanavir provides adequate pharmacokinetic parameters in HIV-1-infected Thai adults. Clin Pharmacol Ther. 2009;85(4):402–8.
Schipani A, Egan D, Dickinson L, et al. Estimation of the effect of SLCO1B1 polymorphisms on lopinavir plasma concentration in HIV-infected adults. Antivir Ther. 2012;17(5):861–8.
Lubomirov R, di Iulio J, Fayet A, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20(4):217–30.
Schipani A, Siccardi M, D’Avolio A, et al. Population pharmacokinetic modeling of the association between 63396C→T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob Agents Chemother. 2010;54(12):5242–50.
Janneh O, Jones E, Chandler B, et al. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007;60(5):987–93.
Janneh O, Owen A, Chandler B, et al. Modulation of the intracellular accumulation of saquinavir in peripheral blood mononuclear cells by inhibitors of MRP1, MRP2, P-gp and BCRP. AIDS. 2005;19(18):2097–102.
Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004;1(1):49–56.
Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20(2):112–20.
Droste JA, Aarnoutse RE, Koopmans PP, et al. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J Acquir Immune Defic Syndr. 2003;32(3):287–91.
Beal SL, Sheiner LB. NONMEM Users Guides. Ellicott City: Icon Development Solutions; 1989–1998.
Karlsson MO, Jonsson EN, Wiltse CG, et al. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26(2):207–46.
Jonsson EN, Karlsson MO. Xpose: an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64.
Moltó J, Barbanoj MJ, Miranda C, et al. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet. 2008;47(10):681–92.
Sale M, Sadler BM, Stein DS. Pharmacokinetic modeling and simulations of interaction of amprenavir and ritonavir. Antimicrob Agents Chemother. 2002;46(3):746–54.
Lu JF, Blaschke TF, Flexner C, et al. Model-based analysis of the pharmacokinetic interactions between ritonavir, nelfinavir, and saquinavir after simultaneous and staggered oral administration. Drug Metab Dispos. 2002;30(12):1455–61.
Kappelhoff BS, Huitema AD, Crommentuyn KM, et al. Development and validation of a population pharmacokinetic model for ritonavir used as a booster or as an antiviral agent in HIV-1-infected patients. Br J Clin Pharmacol. 2005;59(2):174–82.
Vis P, Sekar V, van Schaick E, et al. Development and application of a population pharmacokinetic model of TMC114 in healthy volunteers and HIV-1 infected subjects alter administration of TMC114 in combination with low dose ritonavir [abstract no. 964] 15th meeting of the Population Approach Group in Europe. 2006 June 14–16; Bruges, Belgium.
Huber RD, Gao B, Sidler Pfändler MA, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol. 2007;292(2):C795–806.
Janneh O, Chandler B, Hartkoorn R, et al. Intracellular accumulation of efavirenz and nevirapine is independent of P-glycoprotein activity in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2009;64(5):1002–7.
Siccardi M, D’Avolio A, Baietto L, et al. SLCO3A1 expression is a major determinant of atazanavir PBMC penetration in HIV infected patients. J Int AIDS Soc. 2010;13(Suppl 4):P178.
Acknowledgments
M.V. is supported by FIS (CP04/00121) from the Spanish Health Department in collaboration with l’Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona, and is a member of the CIBERSAM Network. Genotyping was conducted as part of a project funded by the UK Medical Research Council (G0800247). This study was funded by a grant from the “Lluita contra la SIDA” Foundation and by ISCIII-RETIC RD06/006. The research leading to these results has also received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under the project Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN—grant agreement no. 223131). Genotyping was performed in the Wolfson Centre for Personalised Medicine. We thank the patients and their care givers for their participation in this study. We also wish to acknowledge the contribution of Mary Ellen Kerans, who gave her advice on the English language in the final version of the manuscript.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the context of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moltó, J., Xinarianos, G., Miranda, C. et al. Simultaneous Pharmacogenetics-Based Population Pharmacokinetic Analysis of Darunavir and Ritonavir in HIV-Infected Patients. Clin Pharmacokinet 52, 543–553 (2013). https://doi.org/10.1007/s40262-013-0057-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0057-6