Abstract
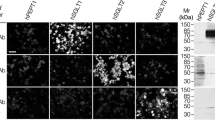
Arginine is a precursor for the synthesis of urea, polyamines, creatine phosphate, nitric oxide and proteins. It is synthesized from ornithine by argininosuccinate synthetase and argininosuccinate lyase and is degraded by arginase, which consists of a liver-type (arginase I) and a non-hepatic type (arginase II). Recently, cDNAs for human and rat arginase II have been isolated. In this study, immunocytochemical analysis showed that human arginase II expressed in COS-7 cells was localized in the mitochondria. Arginase II mRNA was abundant in the rat small intestine and kidney. In the kidney, argininosuccinate synthetase and lyase were immunostained in the cortex, intensely in proximal tubules and much less intensely in distal tubules. In contrast, arginase II was stained intensely in the outer stripes of the outer medulla, presumably in the proximal straight tubules, and in a subpopulation of the proximal tubules in the cortex. Immunostaining of serial sections of the kidney showed that argininosuccinate synthetase and arginase II were collocalized in a subpopulation of proximal tubules in the cortex, whereas only the synthetase, but not arginase II, was present in another subpopulation of proximal tubules. In the liver, all the enzymes of the urea cycle, i.e. carbamylphosphate synthetase I, ornithine transcarbamylase, argininosuccinate synthetase and lyase and arginase I, showed similar zonation patterns with staining more intense in periportal hepatocytes than in pericentral hepatocytes, although zonation of ornithine transcarbamylase was much less prominent. The implications of these results are discussed.
Similar content being viewed by others
References
Aoki, E. & Takeuchi, I.K. (1997) Immunohistochemical localization of arginine and citrulline in rat renal tissue. J. Histochem. Cytochem. 45, 875-81.
Bettuzzi, S., Marinelli, M., Strocchi, P., Cavolani, D. & Corti, A. (1995) Different localization of spermidine/spermine N1-acetyltransferase and ornithine decarboxylase transcripts in the rat kidney. FEBS Lett. 377, 321-24.
Blackshear, P. J., Manzella, J.M., Stumpo, D.J., Wen, L. Huang, J.K., Oyen, O. & Young, W. (1989) High level, cell-specific expression of ornithine decarboxylase transcripts in rat genitourinary tissues. Mol. Endocrinol. 3, 68-78.
Chomczynski, P. & Sacchi, N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal. Biochem. 162, 156-9.
Dhanakoti, S.N., Brosnan, M.E., Herzberg, G.R. & Brosnan, J.T. (1992) Cellular and subcellular localization of enzymes of arginine metabolism in rat kidney. Biochem. J. 282, 369-75.
Dingemanse, M.A., De Jonge, W., De Boer, P., Mori, M., Lamers, W.H. & Moorman, A.F. (1996) Development of the ornithine cycle in rat liver: zonation of a metabolic pathway. Hepatology 24, 407-11.
Gaasbeek, J.J., Lamers, W.H., Moorman, A.F., De Graaf, A., Los, J.A. & Charles, R. (1984) Immuno-histochemical localization of carbamoyl-phosphate synthetase (ammonia) in adult rat liver; evidence for a heterogeneous distribution. J. Histochem. Cytochem. 32, 557-64.
Glass, R.D. & Knox, W.E. (1973) Arginase isozymes of rat mammary gland, liver, and other tissues. J. Biol. Chem. 248, 5785-9.
Gotoh, T., Sonoki, T., Nagasaki, A., Terada, K., Takiguchi, M. & Mori, M. (1996) Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett. 395, 119-22.
Grody, W.W., Kern, R.M., Klein, D., Dodson, A.E., Wissman, P.B., Barsky, S.H. & Cederbaum, S.D. (1993) Arginase deFIciency manifesting delayed clinical sequel and induction of a kidney arginase isozyme. Hum. Genet. 91, 1-5.
Hattori, Y., Campbell, E.B. & Gross, S.S. (1994) Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration or arginine for nitric oxide synthesis. J. Biol. Chem. 269, 9405-8.
Hecker, M., Sessa, W.C., Harris, H.J., Anggard, E.E. & Vane, J.R. (1990) The metabolism of L-arginine and its significance for the biosynthesis of endothelium derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. USA 87, 8612-16.
Herzfeld, A. & Raper, S.M. (1976) The heterogeneity of arginases in rat tissues. Biochem. J. 153, 469-78.
Ikemoto, M., Tabata, M., Miyake, T., Kono, T., Mori, M., Totani, M. & Murachi, T. (1990) Expression of human liver arginase in Escherichia coli. Purification and properties of the product. Biochem. J. 270, 697- 703.
Kanazawa, M., Terada, K., Kato, S. & Mori, M. (1997) HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J. Biochem. 121, 890-5.
Kasahara, M., Matsuzawa, T., Kokubo, M., Gushiken, Y., Tashiro, K., Koide, T., Watanabe, H. & Katunuma, N. (1986) Immunohistochemical localization of ornithine aminotransferase in normal rat tissues by Fab'-horseradish peroxidase conjugates. J. Histochem. Cytochem. 34, 1385-8.
Kato, H., Oyamada, I., Mizutani-Funahashi, M. & Nakagawa, H. (1976) New radioisotopic assays of argininosuccinate synthetase and argininosuccinase. J. Biochem. 79, 945-53.
Kaysen, G.A. & Strecker, H.J. (1973) Purification and properties of arginase of rat kidney. Biochem. J. 133, 779-88.
Levillain, O., Hus, C.A., Morel, F. & Bankir, L. (1989) Production of urea from arginine in pars recta and collecting duct of the rat kidney. Renal Physiol. Biochem. 12, 302-12.
Levillain, O., Hus, C.A., Morel, F. & Bankir, L. (1990) Localization of arginine synthesis along rat nephron. Am. J. Physiol. 259, 916-23.
Matsuzawa, T., Kobayashi, T., Kazuhiro, T. & Kasahara, M. (1994) Changes in ornithine metabolic enzymes induced by dietary protein in small intestine and liver: intestine-liver relationship in ornithine supply to liver. J. Biochem. 116, 721-7.
Mitchell, J.A., Hecker, M. & Vane, J.R. (1990) The generation of L-arginine in endothelial cells is linked to the release of endothelium-derived relaxing factor. Eur. J. Pharmacol. 176, 253-54.
Moorman, A.F., Vermeulen, J.L., Charles, R. & Lamers, W.H. (1989) Localization of ammonia-metabolizing enzymes in human liver: ontogenesis of heterogeneity. Hepatology 9, 367-72.
Moorman, A.F., De Boer, B.P., Evans, D., Charles, R. & Lamers, W.H. (1990) Expression patterns of mRNAs for alpha-fetoprotein and albumin in the developing rat: the ontogenesis of hepatocyte heterogeneity. Histochem. J. 22, 653-60.
Mori, M., Miura, S., Tatibana, M. & Cohen, P.P. (1979) Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc. Natl. Acad. Sci. USA 76, 5071-5.
Morris, S.J. (1992) Regulation of enzymes of urea and arginine synthesis. Annu. Rev. Nutr. 12, 81-101.
Morris, S. J., Sweeney, W.J., Kepka, D.M., O'brien, W.E. & Avner, E.D. (1991) Localization of arginine biosynthetic enzymes in renal proximal tubules and abundance of mRNA during development. Pediatr. Res. 29, 151-4.
Nagasaki, A., Gotoh, T., Takeya, M., Yu, Y., Takiguchi, M., Matsuzaki, H., Takatsuki, K. & Mori, M. (1996) Coinduction of nitric oxide synthase, argininosuccinate synthetase, and argininosuccinate lyase in lipopolysaccharide-treated rats. RNA blot, immunoblot, and immunohistochemical analyses. J. Biol. Chem. 271, 2658-62.
Nebes, V.L. & Morris, S. J. (1988) Regulation of messenger ribonucleic acid levels for five urea cycle enzymes in cultured rat hepatocytes. Requirements for cyclic adenosine monophosphate, glucocorticoids, and on-going protein synthesis. Mol. Endocrinol. 2, 444-51.
Nussler, A.K., Billiar, T.R., Liu, Z.Z. & Morris, S.J. (1994) Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. Implications for regulation of nitric oxide production. J. Biol. Chem. 269, 1257-61.
Ratner, S. & Murakami, M.K. (1980) A new radiochemical assay for argininosuccinase with purified [14C]argininosuccinate. Anal. Biochem. 106, 134-47.
Saheki, T., Yagi, Y., Sase, M., Nakano, K. & Sato, E. (1983) Immunohistochemical localization of argininosuccinate synthetase in the liver of control and citrullinemic patients. Biomed. Res. 4, 235-8.
Skrzypek-Osiecka, I., Robin, Y. & Porembska, Z. (1983) PuriFIcation of rat kidney arginases A1 and A4 and their subcellular distribution. Acta Biochim. Pol. 30, 83-92.
Sonoki, T., Nagasaki, A., Gotoh, T., Takiguchi, M., Takeya, M., Matsuzaki, H. & Mori, M. (1997) Co-induction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J. Biol. Chem. 272, 3689- 93.
Spector, E.B., Jenkinson, C.P., Grigor, M.R., Kern, R.M. & Cederbaum, S.D. (1994) Subcellular location and differential antibody specificity of arginase in tissue culture and whole animals. Int. J. Dev. Neurosci. 12, 337-42.
Takiguchi, M. & Mori, M. (1995) Transcriptional regulation of genes for ornithine cycle enzymes. Biochem. J. 312, 649-59.
Wang, W.W., Jenkinson, C.P., Griscavage, J.M., Kern, R.M., Arabolos, N.S., Byrns, R.E., Cederbaum, S.D. & Ignarro, L.J. (1995) Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochem. Biophys. Res. Commun. 210, 1009-16.
Wu, G.Y. & Brosnan, J.T. (1992) Macrophages can convert citrulline into arginine. Biochem. J. 281, 45-8.
Yano, M., Kanazawa, M., Terada, K., Namchai, C., Yamaizumi, M., Hanson, B., Hoogenraad, N. & Mori, M. (1997) Visualization of mitochondrial protein import in cultured mammalian cells with green fluorescent protein and effects of overexpression of the human import receptor Tom20. J. Biol. Chem. 272, 8459-65.
Yu, Y., Terada, K., Nagasaki, A., Takiguchi, M. & Mori, M. (1995) Preparation of recombinant argininosuccinate synthetase and argininosuccinate lyase: expression of the enzymes in rat tissues. J. Biochem. 117, 952-7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyanaka, K., Gotoh, T., Nagasaki, A. et al. Immunohistochemical Localization of Arginase II and Other Enzymes of Arginine Metabolism in Rat Kidney and Liver. Histochem J 30, 741–751 (1998). https://doi.org/10.1023/A:1003468726969
Issue Date:
DOI: https://doi.org/10.1023/A:1003468726969