Abstract
Purpose. NONMEM was applied to single dose and multiple dose bioavailability data for an immediate release (IR) and a controlled release (CR) dosage form of alprazolam to acquire additional information from the data which are not easily obtainable by traditional means.
Methods. The objective function value (OBJ) and diagnostic plots were used as measures of goodness of fit of the model to the data. A change in the OBJ value of 7.9 was necessary to show statistical significance (p < 0.005) between two models when the two models differed by 1 parameter.
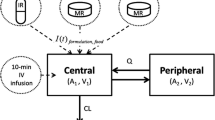
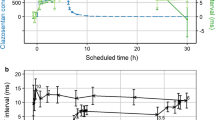
Results. A two-compartment linear model with first-order absorption and elimination best describes the data. Including a lag time, two different rates of absorption (KAIR and KACR), and bioavailability for the CR relative to the IR dosage form significantly improved the fit of the model to the data. Cigarette smoking was associated with a 100% increase in clearance of alprazolam as compared to non-smokers. The higher residual variability observed in this study, where interoccasion variability (IOV) was not initially modeled, could be explained to a large extent by the presence of significant interoccasion variability (IOV).
Conclusions. Since alprazolam has been suggested to be mainly metabolized by the CYP3A4 isozyme in humans, it appears that tobacco could be an inducer of CYP3A4 and/or alprazolam may be metabolized by other isozyme(s) (specifically, CYP1A1/1A2) that are induced by cigarette smoke. The population pharmacokinetic model approach combined with exploratory graphical data analysis is capable of identifying important covariates from well-controlled 'data rich' Phase I studies early in drug development.
Similar content being viewed by others
REFERENCES
D. J. Greenblatt and C. E. Wright. Clin. Pharmacokinet. 24:453–471 (1993).
Physicians' Desk Reference ®, 48th Edition, pages 2456–2459 (1994).
P. D. Garzone and P. D. Kroboth. Clin. Pharmacokinet. 16:337–364 (1989).
L. B. Sheiner, B. Rosenberg, and V. V. Marathe. J. Pharmacokinet. Biopharm. 5:445–479 (1977).
A. D. Graves and I. Chang. J. Pharmacokinet. Biopharm. 18:145–160 (1990).
R. Miller and T. M. Ludden. Eur. J. Clin. Pharmacol. 44:231–235 (1993).
N. Kaniwa, N. Aoyagi, H. Ogata, and M. Ishii. J. Pharm. Sci. 79:1116–1120 (1990).
J. C. Fleishaker, J. P. Phillips, M. G. Eller, and R. B. Smith. J. Clin. Pharmacol. 29:543–549 (1989).
S. L. Beal and L. B. Sheiner. NONMEM Users Guide, Part I–VI, Division of Clinical Pharmacology, University of California, San Francisco, 1979–1992.
R. B. Smith and P. D. Kroboth. Psychopharmacol. 93:105–112 (1987).
M. O. Karlsson and L. B. Sheiner. J. Pharmacokinet. Biopharm. 21:735–750 (1993).
P. O. Maitre, M. Buhrer, D. R. Stanski, and D. Thompson. J. Pharmacokinet. Biopharm. 19:377–384 (1991).
J. W. Mandema, D. Verotta, and L. B. Sheiner. J. Pharmacokinet. Biopharm. 20:511–528 (1992).
M. Davidian and A. R. Galant. J. Pharmacokinet. Biopharm. 20:529–556 (1992).
P. Burtin, E. Jacqz-Aigrain, P. Girard, R. Lenclen, J. F. Magny, P. Betremieux, C. Tehiry, L. Desplanques, and P. Mussat. Clin. Pharmacol. Ther. 56:615–625 (1994).
J. R. Wade, S. L. Beal, and N. C. Sambol. J. Pharmacokinet. Biopharm. 22:165–177 (1994).
E. I. Ette and T. M. Ludden. Pharm. Res. 12:1845–1855 (1995).
S-PLUS (version 3.1), Statistical Sciences Inc., Seattle, Washington, 1992.
J. M. Morgan and K. M. Bray. Clin. Pharmacokinet. 26:292–307, 1994.
C. Kirkwood, A. Moore, P. Hayes, C. L. DeVane, and A. Pelonero. Clin. Pharmacol. Ther. 50:404–409 (1991).
R. B. Smith, P. R. Gwilt, and C. E. Wright. Clin. Pharm. 2:139–143 (1983).
L. L. von Moltke, D. G. Greenblatt, M. M. Cotreau-Bibbo, J. S. Harmatz, and R. I. Shader. Br. J. Clin. Pharmac. 38:23–31 (1994).
N. Yasui, K. Otani, S. Kaneko, T. Ohkubo, T. Osanai, K. Sugawara, K. Chiba, and T. Ishizaki. Clin. Pharmacol. Ther. 59:514–519 (1996).
P. H. Villard, E. Seree, B. Lacarelle, M. C. Therene-Fenoglio, Y. Barra, L. Attolini, B. Bruguerole, A. Durand, and J. Catalin. Biochem. Biophys. Res. Comm. 202:1731–1737 (1994).
R. Agrawal, F. K. Jugert, S. G. Khan, D. R. Bickers, H. F. Merk, and H. Mukhtar. Biochem. Biophys. Res. Comm. 199:1400–1406 (1994).
L. G. Miller. Clin. Pharmacokinet. 17:90–108 (1989).
W. Kalow and B. K. Tang. Clin. Pharmacol. Ther. 49:44–48 (1991).
D. Sesardic, A. R. Boobis, R. J. Edwards, and D. S. Davies. Br. J. Clin. Pharmacol. 26:363–372 (1988).
K. Brøsen, E. Skjelbo, B. B. Rasmussen, H. E. Poulsen, and S. Loft. Biochem. Pharmacol. 45:1211–1214 (1993).
B. B. Rasmussen, J. Maenpaa, O. Pelkonen et al. Br. J. Clin. Pharmacol. 39:151–159 (1995).
L. L. von Moltke, D. J. Greenblatt, M. H. Court, S. X. Duan, J. S. Harmatz, and R. I. Shader. J. Clin. Psychopharmacol. 15:125–131 (1995).
J. C. Fleishaker and L. K. Hulst. Eur. J. Clin. Pharmacol. 46:35–39 (1994).
P. Periti, T. Mazzei, E. Mini, and A. Novelli. Clin. Pharmacokinet. 23:106–131 (1992).
M. A. Sarkar and B. J. Jackson. Drug Metab. Dispos. 22:827–834 (1994).
S. A. Wrighton and J. C. Stevens. Crit. Rev. Toxicol. 22:1–21 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hossain, M., Wright, E., Baweja, R. et al. Nonlinear Mixed Effects Modeling of Single Dose and Multiple Dose Data for an Immediate Release (IR) and a Controlled Release (CR) Dosage Form of Alprazolam. Pharm Res 14, 309–315 (1997). https://doi.org/10.1023/A:1012041920119
Issue Date:
DOI: https://doi.org/10.1023/A:1012041920119