Abstract
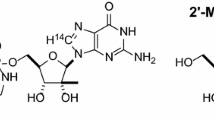
Purpose. A series of prodrugs designed to enhance the oral bioavailability of the antiretroviral agent 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA; 1) have been synthesized, including a bis-(acyloxymethyl) ester 2 and a series of bis-(alkoxycarbonyloxymethyl) esters 3-9. The in vitro biological stability andin vivo pharmacokinetics of these prodrugs were evaluated to support selection of a prodrug candidate for clinical evaluation.
Methods. The in vitrobiological stability of the prodrugs was examined in dog tissues (intestinal homogenate, plasma and liver homogenate). The apparent half-lives were determined based on the disappearance of prodrug using reverse-phase HPLC with UV detection. Oral bioavailability of PMPA from each prodrug was determined in fasted beagle dogs. Concentrations of PMPA in plasma were determined by HPLC following fluorescence derivatization. Data for prodrugs were compared to historical data for intravenous PMPA.
Results. All prodrugs were rapidly hydrolyzed in dog plasma and tissues (t1/2 < 60 min). In fasted beagle dogs, bis-[(pivaloyloxy)methyl] PMPA (bis-POM PMPA) 2 had the highest oral bioavailability as PMPA (37.8 ± 5.1%). The oral bioavailabilities of PMPA from bis-(alkoxycarbonyloxymethyl) esters ranged from 16.0% to 30.7% and PMPA was the major metabolite formed.
Conclusions. There was a correlation between oral bioavailability and intestinal stability of bis-(alkoxycarbonyloxymethyl) ester prodrugs (r2 = 0.96). Lipophilicity (log P) was not a good predictor of oral bioavailability. The most labile prodrugs in dog intestinal homogenates, bis-(n-butyloxycarbonyloxymethyl) PMPA 5 and bis-(neo-pentyloxycarbonyloxymethyl) PMPA 8 (t1/2 < 5 min) had the lowest oral bioavailabilities. Based on good oral bioavailability (30.1%), chemical and intestinal stability bis-(isopropyloxycarbonyloxymethyl) PMPA (bis-POC PMPA) 4 was selected as a candidate for clinical evaluation.
Similar content being viewed by others
REFERENCES
J. Balzarini, L. Naesens, P. Herdewijn, I. Rosenberg, A. Holy, R. Pauwels, M. Baba, D. G. Johns, and E. De Clercq. Proc. Natl. Acad. Sci. USA 86:332–336 (1989).
M. Wachsman, B. G. Petty, K. C. Cundy, H. S. Jaffe, P. E. Fisher, A. Pastelak, and P. S. Lietman. Antiviral Res. 29:153–161 (1996).
K. C. Cundy, P. Barditch-Crovo, R. E. Walker, A. C. Collier, D. Ebeling, J. Toole, and H. S. Jaffe. Antimicrob Agents Chemother. 39:2401–2405 (1995).
J.-P. Shaw, M. S. Louie, V. V. Krishnamurthy, M. N. Arimilli, R. J. Jones, Alison M. Bidgood, W. A. Lee, and K. C. Cundy. Drug Metab Dispos. 25:361–366 (1997).
W. A. Lee, J.-P. Shaw, C. Sueoka, K. C. Cundy, N. Bischofberger, S. Lacy, and S. Swaminathan. 4th Conferences on Retroviruses and Opportunistic Infection, January, Washington, D.C. (Abstract) (1997).
H. T. Serafinowska, R. J. Ashton, S. Bailey, M. R. Harnden, S. M. Jackson, and D. Sutton. J. Med. Chem. 38:1372–1379 (1995).
P. Barditch-Crovo, J. Toole, C. W. Hendrix, K. C. Cundy, D. Ebeling, H. S. Jaffe, and P. S. Lietman. J. Infectious Dis. 176:406–413 (1997).
J. Balzarini, A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. Antimicrob. Agents Chemother. 37:332–338 (1993).
C.-C. Tsai, K. E. Follis, A. Sabo, T. W. Beck, R. F. Grant, N. Bischofberger, R. E. Benveniste, and R. Black. Science. 270:1197–1199 (1995).
N. Bischofberger, C. Miller, Z. Rosenberg. Presented at Ninth International Conference on Antiviral Research. Fukushima, Japan, 1996.
K. K. A. Van Rompay, J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, N. Bischofberger, and N. C. Pedersen. Antimicrob. Agents Chemother. 40:2586–2591 (1996).
P. Barditch-Crovo, S. Deeks, J. Kahn, I. Redpath, A. Smith, F. Hwang, N. Hellmann, K. Cundy, J. Rooney, and P. Lietman. Tenth International Conference on Antiviral Research, Atlanta, Georgia (Abstract) (1997).
C. M. Sueoka, L. Griffin, and J.-P. Shaw. unpublished results.
J. Alexanders, R. Cargill, S. R. Michelson, and H. Schwam. J. Med. Chem. 31:318–322 (1988).
M. Safadi, R. Oliyai, and V. J. Stella. Pharm. Res. 10:1350–1355 (1993).
M. N. Arimilli, C. U. Kim, N. Bischofberger, J. Dougherty, A. Mulato, J.-P. Shaw, C. Sueoka, R. Oliyai, K. C. Cundy, and W. A. Lee. Antimicrob. Agents Chemother. (in press).
G. Powis. Drug Metabolism Review. 20:379–394 (1989).
J. Russell, D. Marrero, V. J. Whiterock, L. J. Klunk, and J. E. Starrett. J. Chromatogr. (Netherlands). 572:321–326 (1991).
PCNONLIN® Version 4.2. Statistical Consultants, Inc., Lexington, Kentucky.
R. E. Notari. In “Design of Prodrugs” (H. Bundgaard, ed.) p 135–156. Elsevier Science Publishers (1985).
StatView® Version 4.0. Abacus Concepts, Inc., Berkeley, California.
K. Krisch. In “The Enzymes”, 3rd ed. Vol. 5a (P. D. Boyer, ed.) p 43–69. Academic Press (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shaw, JP., Sueoka, C.M., Oliyai, R. et al. Metabolism and Pharmacokinetics of Novel Oral Prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in Dogs. Pharm Res 14, 1824–1829 (1997). https://doi.org/10.1023/A:1012108719462
Issue Date:
DOI: https://doi.org/10.1023/A:1012108719462