Abstract
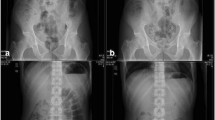
Captopril has been administered to eight healthy male subjects by means of a pulsatile delivery system that was designed to release the drug in the colonic region of the intestine. The gastrointestinal transit and pulsatile release were followed using gamma scintigraphy. A pulsatile capsule system with release after a nominal 5-hr period was found to perform reproducibly in vitro and in vivo. In six of the eight subjects, the drug was delivered to the colon, and in the remaining two subjects, to the terminal ileum. Measurable blood levels of free captopril were found in three subjects. Variable instability of the drug in the distal intestine is suggested as a possible reason for the lack of absorption of the drug in the majority of subjects.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Similar content being viewed by others
REFERENCES
R. N. Brogen, P. A. Todd, and E. M. Sorkin. Captopril: An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and congestive heart failure. Drugs 36:540–600 (1988).
P. J. Worland, O. H. Drummer, and B. Jarrott. Gastric and intestinal absorption of captopril in acutely and chronically treated rats: Comparison with salicylic acid. J. Pharm. Sci. 73:1755–1758 (1984).
S. S. Davis. The design and evaluation of controlled release delivery systems. J. Control. Release 2:27–38 (1985).
S. S. Davis, N. Washington, G. D. Parr, A. H. Short, V. A. John, P. Lloyd, and S. M. Walker. Relationship between the rate of appearance of oxprenolol in the systemic circulation and the location of an oxprenolol Oros 16/260 drug delivery system within the gastrointestinal tract as determined by scintigraphy. Br. J. Clin. Pharmacol. 26:435–443 (1988).
S. S. Davis. Evaluation of gastrointestinal transit and release characteristics of drugs. In Advances in Drug Delivery, P. Johnson and G. Lloyd-Jones (eds.), Ellis Horwood, Chichester, 1987, pp. 164–179.
J. G. Hardy, S. S. Davis, R. Khosla, and C. S. Robertson. Gastrointestinal transit of small tablets in patients with ulcerative colitis. Int. J. Pharm. 48:79–82 (1988).
S. S. Davis and J. W. Fara. Osmotic pumps. In Drug Delivery to the Gastrointestinal Tract, J. G. Hardy, S. S. Davis, and C. G. Wilson (eds.), Ellis Horwood, Chichester, 1989, pp. 97–109.
A. Rashid. Dispensing Device, British Patent Application 2230441A, 15 Feb. 1990.
S. S. Davis, J. G. Hardy, and J. W. Fara. The intestinal transit of pharmaceutical dosage forms. Gut 27:886–892 (1986).
T. Funke, E. Ivashkiv, M. F. Walley, and A. I. Cohen. Gas chromatography/selective ion monitoring mass spectrometric determination of captopril in human blood. Anal. Chem. 52:1086–1089 (1980).
H. M. Park, S. M. Chernish, B. D. Rosenek, R. L. Brunelle, B. Hargrove, and H. N. Wellman. Gastric emptying of enteric coated tablets. Dig. Dis. Sci. 29:207–212 (1984).
C. F. Code and J. A. Marlett. The interdigestive myoelectric complex of the stomach and small bowel of dogs. J. Physiol. 246:289–309 (1975).
V. A. John, P. A. Shotton, J. Moppert, and W. Theobald. Gastrointestinal transit of Oros drug delivery systems in healthy volunteers: A short report. Br. J. Clin. Pharmacol. 19:2035–2065 (1985).
M. Hu and G. L. Amidon. Passive and carrier mediated intestinal absorption components of captopril. J. Pharm. Sci. 77:1007–1011 (1988).
J. Brennan, D. O'Donnell, M. Zinny, N. Jain, E. Ivashkiv, and M. Arnold. The comparative bioavailability of captopril after colonic infusion and oral administration in healthy volunteers. Proc. 92nd Meet. Amer. Soc. Clin. Pharmac. Ther., San Antonio, 1991, p. 9.
T. Komai, T. Ikeda, K. Kawai, E. Kameyama, and H. Shendo. In-vitro studies on the metabolic pathway of SQ 14225 (captopril) and mechanism of mixed disulfide formation. J. Pharmacobio-dynam. 4:677–684 (1981).
K. K. Wong, S.-J. Lan, and B. H. Migdalof. In-vitro biotransformations of (14C) captopril in the blood of rats, dogs and humans. Biochem. Pharmacol. 30:2643–2650 (1981).
J. H. Cummings. Colonic absorption: the importance of short chain fatty acids in man. Scand. J. Gastroenterol. 19(Suppl. 93):89–99 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilding, I.R., Davis, S.S., Bakhshaee, M. et al. Gastrointestinal Transit and Systemic Absorption of Captopril from a Pulsed-Release Formulation. Pharm Res 9, 654–657 (1992). https://doi.org/10.1023/A:1015806211556
Issue Date:
DOI: https://doi.org/10.1023/A:1015806211556