Abstract
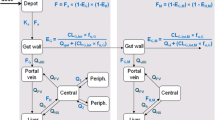
Purpose. The relative contribution of the intestinal mucosa, liver and lung to the in vivo disposition of propofol in the rat was investigated.
Methods. Propofol (4.9–5.1 mg · kg−l) was administered to groups of rats (n = 4) via the intra-arterial, intravenous, hepatic portal venous and oral routes. The AUC's of propofol were estimated and the fractions of the administered dose escaping first pass metabolism by the gut wall (fG), liver (fH) and lung (fL) were calculated. In addition, transport experiments were carried out using Caco-2 cell monolayers to rule out the possibility that intestinal permeability is limiting the oral absorption of propofol.
Results. Values for fG, fH and fL were the following: 0.21 ± 0.07, 0.61 ± 0.13, and 0.82 ± 0.09, respectively. The apparent permeability coefficient of propofol across Caco-2 cell monolayers was 24.2 ± 0.3 × 10−6 cm · sec−1, which is similar to the apparent permeability coefficient obtained for propranolol (30.7 ± 1.7 × 10−6 cm · sec−1), a compound known to easily cross the intestinal epithelial membranes. The formation of propofol glucuronide, a major metabolite of propofol, could not be demonstrated during the flux experiments across the Caco-2 cell monolayers.
Conclusions. The intestinal mucosa is the main site of first pass metabolism following oral administration of propofol in the rat. Intestinal metabolism could therefore also contribute to the systemic clearance of propofol.
Similar content being viewed by others
REFERENCES
P. J. Simons, I. D. Cockshott, E. J. Douglas, E. A. Gordon, S. Knott, and R. J. Ruane. Species differences in blood profiles, metabolism and excretion of 14C-propofol after intravenous dosing to rat, dog and rabbit. Xenobiotica 21:1243–1256 (1991).
P. J. Simons, I. D. Cockshott, E. J. Douglas, E. A. Gordon, K. Hopkins, and M. Rowland. Disposition in male volunteers of a subanaesthetic intravenous dose of an oil in water emulsion of 14C-propofol. Xenobiotica 18:429–440 (1988).
H. M. Bryson, B. R. Fulton, and D. Faulds. Propofol: an update of its use in anaesthesia and conscious sedation. Drugs 50:513–559 (1995).
H. Lange, H. Stephan, H. Rieke, M. Kellermann, H. Sonntag, and Bircher J. Hepatic and extrahepatic disposition of propofol in patients undergoing coronary bypass surgery. Br. J. Anaesth. 74:46–49 (1995).
P. Veroli, B. O'Kelly, F. Bertrand, J. H. Trouvin, R. Farinotti, and C. Ecoffey. Extrahepatic metabolism of propofol in man during the anhepatic phase of orthotopic liver transplantation. Br. J. Anaesth. 68:183–186 (1992).
I. J. Hidalgo, T. J. Raub, and R. T. Borchardt. Characterization of the human colon cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749 (1989).
P. Artursson. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79:476–482 (1990).
M. A. Hurni, A. B. J. Noach, M. C. M. Blom-Roosemalen, A. G. De Boer, J. F. Nagelkerke, and D. D. Breimer. Permeability enhancement in Caco-2 cell monolayers by sodium salicylate and sodium tauro-dihydrofusidate: assessment of effect-reversibility and imaging on transport routes by confocal laser scanning microscopy. J. Pharmacol. Exp. Ther. 267:942–950 (1993).
G. F. Plummer. Improved method for the determination of propofol in blood by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 421:171–176 (1987).
A. A. Raoof, L. J. Van Obbergh, J. de Ville de Goyet, and R. K. Verbeeck. Extrahepatic glucuronidation of propofol in man: possible contribution of gut wall and kidney. Eur. J. Clin. Pharmacol.,50:91–96 (1996).
M. K. Cassidy and J. B. Houston. In vivo assessment of extrahepatic conjugative metabolism in first pass effects using the model compound phenol. J. Pharm. Pharmacol. 32:57–59 (1980).
M. Capiello, L. Giuliani, and G. M. Pacifici. Distribution of UDP-glucuronosyltransferase and its endogenous substrate uridine 5′-diphosphoglucuronic acid in human tissues. Eur. J. Clin. Pharmacol. 41:345–350 (1991).
P. S. Leppert and J. A. Fix. Use of the everted intestinal rings for in vitro examination of oral absorption potential. J. Pharm. Sci. 83:976–981 (1994).
B. H. Stewart, O. H. Chan, R. H. Lu, E. L. Reyner, H. L. Schmid, H. W. Hamilton, B. A. Steinbaugh, and M. D. Taylor. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm. Res. 12:693–699 (1995).
S. Bjorge, K. L. Hamelehle, R. Homan, S. E. Rose, D. A. Turluck, and D. Scott Wright. Evidence for glucuronide conjugation of p-nitrophenol in the Caco-2 cell model. Pharm. Res. 8:1441–1443 (1991).
P. J. Chikhale and R. T. Borchardt. Metabolism of L-α-methyldopa in cultured human intestinal epithelial (Caco-2) cell monolayers. Drug Metab. Dispos. 22:592–600 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raoof, A.A., Augustijns, P.R. & Verbeeck, R.K. In Vivo Assessment of Intestinal, Hepatic, and Pulmonary First Pass Metabolism of Propofol in the Rat. Pharm Res 13, 891–895 (1996). https://doi.org/10.1023/A:1016057229478
Issue Date:
DOI: https://doi.org/10.1023/A:1016057229478