Abstract
Purpose. To investigate whether Madin-Darby canine kidney cells transfected with the human MRP2 gene (MDCK-MRP2) are a good model of the human intestinal mucosa.
Methods. MRP2 expression in Caco-2 cells was compared with the expression of this efflux transporter in MDCK-wild type (MDCK-WT) and MDCK-MRP2 cells using Western blotting methods. The polarized efflux activities of MRP2 in the MDCK-MRP2, MDCK-WT, MDCK cells transfected with the human MDR1 gene (MDCK-MDR1), and Caco-2 cells were compared using vinblastine as a substrate. Apparent Michaelis-Menten constants (KM, Vmax) for the efflux of vinblastine in Caco-2 and MDCK-MRP2 cells were determined in the presence of GF120918 (2 μM), which inhibits P-glycoprotein but does not affect MRP2. Apparent inhibitory constants (KI) of known substrates/inhibitors of MRP2 were determined by measuring their effects on the efflux of vinblastine in these cell lines.
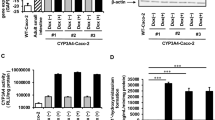
Results. MDCK-MRP2 cells expressed higher levels of MRP2 than MDCK-WT and Caco-2 cells as measured by Western blotting technique. Two isoforms of MRP2 expressed in Caco-2 and MDCK cells migrated at molecular weights of 150 kD and 190 kD. In MDCK-MRP2 cells, the 150 kD isoform appeared to be overexpressed. MDCK-MRP2 cell monolayers exhibited higher polarized efflux of vinblastine than Caco-2 and MDCK-WT cell monolayers. KM values for vinblastine in Caco-2 and MDCK-MRP2 cells were determined to be 71.8 ± 11.6 and 137.3 ± 33.6 μM, respectively, and Vmax values were determined to be 0.54 ± 0.03 and 2.45 ± 0.31 pmolcm−2s−1, respectively. Known substrates/inhibitors of MRP2 showed differences in their ability to inhibit vinblastine efflux in Caco-2 cells as compared to MDCK-MRP2 cells
Conclusions. These data suggest that MDCK-MRP2 cells overexpress only the 150 kD isoform of MRP2. The 190 kD isoform, which was also found in Caco-2 cells and MDCK-WT cells, was present in MDCK-MRP2 cells but not over expressed. The apparent kinetics constants and affinities of some MRP2 substrates were different in Caco-2 cells and MDCK-MRP2 cells. These differences in substrate activity could result from differences in the relative expression levels of the MRP2 isoforms present in Caco-2 cells and MDCK-MRP2 cells and/or differences in the partitioning of substrates in these two cell membrane bilayers.
Similar content being viewed by others
REFERENCES
P. Borst, R. Evers, M. Kool, and J. Wijnholds. The multidrug resistance protein family. Biochim Biophys. Acta. 1461:347–357 (1999).
J. Konig, A. T. Nies, Y. Cui, I. Leier, and D. Keppler. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1461:377–394 (1999).
P. L. Jansen, W. H. Peters, and W. H. Lamers. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 5:573–579 (1985).
C. G. Dietrich, D. R. de Waart, R. Ottenhoff, I. G. Schoots, and R. P. Elferink. Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol. Pharmacol. 59:974–980 (2001).
R. Evers, M. Kool, L. van Deemter, H. Janssen, J. Calafat, L. C. Oomen, C. C. Paulusma, R. P. Oude Elferink, F. Baas, A. H. Schinkel, and P. Borst. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J. Clin. Invest. 101:1310–1319 (1998).
P. Artursson, K. Palm, and K. Luthman. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46:27–43 (2001).
R. T. Borchardt. The application of cell culture systems in drug discovery and development. J. Drug Target. 3:179–182 (1995).
K. I. Hosoya, K. J. Kim, and V. H. Lee. Age-dependent expression of P-glycoprotein gp170 in Caco-2 cell monolayers. Pharm. Res. 13:885–890 (1996).
U. K. Walle, A. Galijatovic, and T. Walle. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 58:431–438 (1999).
P. S. Burton, R. A. Conradi, A. R. Hilgers, and N. F. Ho. Evidence for a polarized efflux system for peptides in the apical membrane of Caco-2 cells. Biochem. Biophys. Res. Commun. 190: 760–766 (1993).
J. Taipalensuu, H. Tornblom, G. Lindberg, C. Einarsson, F. Sjoqvist, H. Melhus, P. Garberg, B. Sjostrom, B. Lundgren, and P. Artursson. Correlation of gene expression of ten drug efflux proteins of the atp-binding cassette transporter family in normal human jejunum and in human intestinal epithelial caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 299:164–170 (2001).
A. Soldner, L. Z. Benet, E. Mutschler, and U. Christians. Active transport of the angiotensin-II antagonist losartan and its main metabolite EXP 3174 across MDCK-MDR1 and caco-2 cell monolayers. Br. J. Pharmacol. 129:1235–1243 (2000).
J. Hunter, M. A. Jepson, T. Tsuruo, N. L. Simmons, and B. H. Hirst. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J. Biol. Chem. 268:14991–14997 (1993).
J. D. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J. W. Tolan, H. E. Selick, and J. R. Grove. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 88:28–33 (1999).
R. Evers, N. H. Cnubben, J. Wijnholds, L. van Deemter, P. J. van Bladeren, and P. Borst. Transport of glutathione prostaglandin A Tang, Horie, and Borchardt 778 conjugates by the multidrug resistance protein 1. FEBS Lett. 419: 112–116 (1997).
K. A. Lentz, J. W. Polli, S. A. Wring, J. E. Humphreys, and J. E. Polli. Influence of passive permeability on apparent P-glycoprotein kinetics. Pharm. Res. 17:1456–1460 (2000).
F. Tang, H. Kazutoshi, and R. T. Borchardt. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm. Res. 19:765–777 (2002).
J. Gao, E. D. Hugger, M. S. Beck-Westermeyer, and R. T. Borchardt. In A. Doyle, J. B. Griffiths, D. J. Newell (eds.), Current Protocols in Pharmacology, Vol. 7.2, John Wiley & Sons, Inc., New York, 2000 pp. 1–23.
J. Gao, O. Murase, R. L. Schowen, J. Aube, and R. T. Borchardt. A functional assay for quantitation of the apparent affinities of ligands of P-glycoprotein in Caco-2 cells. Pharm. Res. 18:171–176 (2001).
M. C. Cabot, A. E. Giuliano, T. Y. Han, and Y. Y. Liu. SDZ PSC 833, the cyclosporine A analogue and multidrug resistance modulator, activates ceramide synthesis and increases vinblastine sensitivity in drug-sensitive and drug-resistant cancer cells. Cancer Res. 59:880–885 (1999).
K. Utsunomiya, J. R. Ballinger, M. Piquette-Miller, A. M. Rauth, W. Tang, Z. F. Su, and M. Ichise. Comparison of the accumulation and efflux kinetics of technetium-99m sestamibi and technetium-99m tetrofosmin in an MRP-expressing tumour cell line. Eur. J. Nucl. Med. 27:1786–1792 (2000).
S. P. Letrent, G. M. Pollack, K. R. Brouwer, and K. L. Brouwer. Effects of a potent and specific P-glycoprotein inhibitor on the blood-brain barrier distribution and antinociceptive effect of morphine in the rat. Drug Metab. Dispos. 27:827–834 (1999).
C. Tanaka, R. Kawai, and M. Rowland. Dose-dependent pharmacokinetics of cyclosporin A in rats: events in tissues. Drug Metab. Dispos. 28:582–589 (2000).
A. J. Smith, U. Mayer, A. H. Schinkel, and P. Borst. Availability of PSC833, a substrate and inhibitor of P-glycoproteins, in various concentrations of serum. J. Natl. Cancer Inst. 90:1161–1166 (1998).
A. T. Nies, T. Cantz, M. Brom, I. Leier, and D. Keppler. Expression of the apical conjugate export pump, Mrp2, in the polarized hepatoma cell line, WIF-B. Hepatology. 28:1332–1340 (1998).
K. W. Bock, T. Eckle, M. Ouzzine, and S. Fournel-Gigleux. Coordinate induction by antioxidants of UDP-glucuronosyltransferase UGT1A6 and the apical conjugate export pump MRP2 (multidrug resistance protein 2) in Caco-2 cells. Biochem. Pharmacol. 59:467–470 (2000).
G. Jedlitschky, I. Leier, U. Buchholz, J. Hummel-Eisenbeiss, B. Burchell, and D. Keppler. ATP-dependent transport of bilirubin glucuronides by the multidrug resistance protein MRP1 and its hepatocyte canalicular isoform MRP2. Biochem. J. 327:305–310 (1997).
R. Evers, G. J. Zaman, L. van Deemter, H. Jansen, J. Calafat, L. C. Oomen, R. P. Oude Elferink, P. Borst, and A. H. Schinkel. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Invest. 97:1211–1218 (1996).
V. Ling, N. Kartner, T. Sudo, L. Siminovitch, and J. R. Riordan. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat. Rep. 67:869–874 (1983).
Y. Romsicki and F. J. Sharom. Interaction of P-glycoprotein with defined phospholipid bilayers: a differential scanning calorimetric study. Biochemistry. 36:9807–9815 (1997).
Y. Romsicki and F. J. Sharom. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 38:6887–6896 (1999).
J. Ferte. Analysis of the tangled relationships between P-glycoprotein-mediated multidrug resistance and the lipid phase of the cell membrane. Eur. J. Biochem. 267:277–294 (2000).
K. Tanaka, M. Hirai, Y. Tanigawara, K. Ueda, M. Takano, R. Hori, and K. Inui. Relationship between expression level of P-glycoprotein and daunorubicin transport in LLC-PK1 cells transfected with human MDR1 gene. Biochem. Pharmacol. 53:741–746 (1997).
F. Hyafil, C. Vergely, P. Du Vignaud, and T. Grand-Perret. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 53:4595–4602 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, F., Horie, K. & Borchardt, R.T. Are MDCK Cells Transfected with the Human MRP2 Gene a Good Model of the Human Intestinal Mucosa?. Pharm Res 19, 773–779 (2002). https://doi.org/10.1023/A:1016192413308
Issue Date:
DOI: https://doi.org/10.1023/A:1016192413308