Abstract
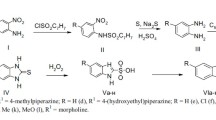
A new type of ultra-short acting β-blocker which might prove advantageous in treating acute arrhythmias was designed, synthesized and investigated. Based on the soft drug “inactive metabolite approach,” the inactive phenylacetic acid metabolite of both metoprolol and atenolol was reactivated by esterification with sulfur-containing aliphatic alcohols. Since the sulfur-containing moieties are labile to the ubiquitous esterases, the new compounds should be inactivated by a one step enzymatic cleavage back to the inactive phenylacetic acid derivative. Pharmacological and pharmacokinetic profiles of the new compounds were evaluated in rats and rabbits. Isoproterenol-induced tachycardia was inhibited with short-term infusion of each compound. This tachycardia blocking effect rapidly disappeared upon termination of infusion, while β-blocking activity was 2–4-fold longer after comparable doses of the short-acting β-blocker, esmolol. The rapid recovery from the β-receptor blockade is believed due to fast hydrolysis of the soft drugs in the body. This is supported from in vitro results showing the tl/2 of esmolol is about 10-fold longer than the new soft drugs in rat, rabbit, dog and human blood. Hydrolysis studies in phosphate buffered solutions indicated that the esters are labile to base-catalyzed hydrolysis. However, the relative t1/2 values measured in biological media compared to phosphate buffered solution clearly support rapid enzymatic cleavage of the soft drugs. Interestingly, one of the soft β-blockers, the sulfonyl ester derivative, showed a unique property of exhibiting good β-receptor blocking activity without significant hypotensive action.
Similar content being viewed by others
REFERENCES
W. H. Frishman, J. Stormand, M. Kirschner, M. Poland, N. Klein, S. Halprin, T. H. LeJemtel, M. Kram and E. H. Sonnenblick. Labetalol Therapy in Patients With Systemic Hypertension and Angina Pectoris: Effects of Combined Alpha and Beta Adrenoceptor Blockade. Am. J. Cardiol. 48:917–928 (1981).
J. G. Gerber and A. S. Nies. Beta-Adrenergic Blocking Drugs. Annu. Rev. Med. 36:145–164 (1985).
D. G. McDevit. Adrenoceptor Blocking Drugs: Clinical Pharmacology and Therapeutic Use. Drugs 17:267–288 (1979).
D. J. Greenblatt and J. Koch-Weser. Adverse Reactions to β-Adrenergic Receptor Blocking Drugs: A Report from Boston Collaborative Drug Surveillance Program. Drugs 7:118–129 (1974).
H. M. Beumer. Adverse Effects of β-Adrenergic Receptor Blocking Drugs on Respiratory Function. Drugs 7:130–138 (1974).
W. Frishman, R. Silverman, J. Strom, U. Elkayam and E. Sonnenblick. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 4. Adverse effects. Choosing a β-adrenoceptor blocker. Am. Heart J. 98,2:256–262 (1979).
W. Frishman and R. Siverman. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 3. Adverse effects. Choosing a β-adrenoceptor blocker. Am. Heart J. 98:119–131 (1979).
V. S. Murphy and W. H. Frishman. Controlled Beta-receptor Blockade with Esmolol and Flestolol. Pharmacotherapy, 5,3:168–1821 (1988).
W. H. Frishman, V. H. Murphy, and J. A. Storm. Ultra-Short-Acting β-Adrenergic Blockers. Med. Clin. of North America, 72:359–372 (1988).
N. Bodor, Y. Oshiro, T. Loftsson, M. Katovich, and W. Caldwell. Soft Drugs VI. The Application of the Inactive Metabolite Approach for Design of Soft β-Blockers. Pharm. Res. 3:120–124 (1984).
P. J. Machin, D. N. Hurst, and J. M. Osbond. β-Adrenoceptor Activity of the Stereoisomer of the Bufuraolol Alcohol and Ketone Metabolites. J. Med. Chem. 28:1648–1651 (1985).
N. Bodor. Prodrugs versus soft drugs. In H. Bundgaard (ed). Design of Prodrugs. Elsevier, Chap. 11, p 333–353 (1985).
N. Bodor. Novel Approaches to the Design of Safer Drugs: Soft Drugs and Site Specific Chemical Delivery System. In Advances in Drug Research, 13, Ed: B. Testa, Academic Press, London, 255–331 (1987).
N. Bodor, A. Elkoussi, M. Kano, and M. Khalifa. Soft Drugs. 7. Soft β-Blockers for Systemic and Ophthalmic Use. J. Med. Chem. 81:1651–1656 (1988).
N. Bodor and A. Elkoussi. Novel ‘soft’ β-blockers as potential safe antiglaucoma agents. Current Eye Research 7:369–374 (1988).
P. W. Erhardt, C. M. Woo, R. J. Gorzynski, and W. G. Anderson. Ultra-Short-Acting β-Adrenergic Blocking Agent. 1. (Aryloxy)propanolamines Containing Esters in the Nitrogen Substituent. J. Med. Chem. 25:1402–1407 (1982).
P. W. Erhardt, C. M. Woo, W. G. Anderson, and R. J. Gorzynski. Ultra-Short-Acting β-Adrenergic Blocking Agent. 2. (Aryloxy)propanolamines Containing Esters on the Aryl Function. J. Med. Chem. 25:1408–1412 (1982).
T. Loftsson, J. Kaminski, and N. Bodor. Improved Delivery through Biological Membranes IX: Kinetics and Metabolism of Hydrolysis of Methylsulfinylmethyl 2-Acetoxybenzoate and Related Aspirin Prodrugs. J. Pharm. Sci. 70,7:750–755 (1981).
T. Loftsson and N. Bodor. Improved Delivery through Biological Membranes X: Percutaneous Absorption and Metabolism of Methylsulfinylmethyl 2-Acetoxybenzoate and Related Aspirin Prodrugs. J. Pharm. Sci. 70,7:756–758 (1981).
P. Turlapaty, A. Laddu, V. S. Merphy, B. Singh, and R. Lee. Esmolol: A Titratable Short-Acting Intravenous Beta-Blocker for Acute Critical Care Settings. Am. Heart J. 114:866–885 (1987).
P. Benfield and E. M. Sorkin. Esmolol. A Preliminary Review of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 33:392–412 (1987).
D. M. Angran, N. J. Schultz, and V. H. Tschida. Esmolol hydrochloride: An ultrashort-acting, β-adrenergic blocking agent. Clinical Pharmacy 5:288–303 (1986).
L. G. Wade, J. M. Gerdes, and R. P. Wirth. Protection of Carboxylic Acids as Methylthiomethyl Esters. Tet. Lett. 8:731–732 (1978).
A. Arfwidsson, K. O. Borg, K. Hoffman, and I. Skanberg. Metabolism of Metoprolol in the Rat in vitro and in vivo. Xenobiotica 6,11:697–711 (1976).
T. Loftsson, J. Kaminski, and N. Bodor. Improved Delivery through Biological Membranes VIII: Design, Synthesis, and In Vivo Testing of True Prodrugs of Aspirin. J. Pharm. Sci. 70,7:743–749 (1981).
C. Y. Quon and H. F. Stampli. Biochemical Properties of Blood Esmolol Esterase. Drug Meta. Dispo. 13,4:420–424 (1985).
N. Weiner. Norepinephrine, Epinephrine, and The Symphatomimetic Amines. In L. S. Goodman, A. G. Gilman, T. W. Rall, and F. Murad (eds). The Pharmacological Basis of Therapeutics. New York. 1990. pp 201–202.
B. E. Bleski. Esmolol. Conn. Med. 51:669–671 (1987).
P. Turlapaty, A. Laddu, V. S. Murphy, B. Singh, and R. Lee. Esmolol: A Titratable Short-Acting Intravenous Beta-Blocker for Acute Critical Care Setting. Am. Heart J. 114:866–885 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, HS., Wu, WM. & Bodor, N. Soft Drugs. XX. Design, Synthesis, and Evaluation of Ultra-Short Acting Beta-Blockers. Pharm Res 12, 329–336 (1995). https://doi.org/10.1023/A:1016283930696
Issue Date:
DOI: https://doi.org/10.1023/A:1016283930696