Abstract
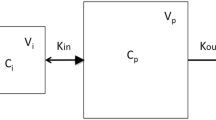
The dispersion model (DM) is a stochastic model describing the distribution of blood-borne substances within organ vascular beds. It is based on assumptions of concurrent convective and random-walk (pseudodiffusive) movements in the direction of flow, and is characterized by the mean transit time \(\left( {\overline {\text{t}} } \right)\) and the dispersion number (inverse Peclet number), DN. The model is used with either closed (reflective) boundary conditions at the inflow and the outflow point (Danckwerts conditions) or a closed condition at the inflow and an open (transparent) condition at the outflow (mixed conditions). The appropriateness of DM was assessed with outflow data from single-pass perfused rat liver multiple indicator dilution (MID) experiments, with varying lengths of the inflow and outflow catheters. The studies were performed by injection of bolus doses of 51Cr-labeled red blood cells (vascular indicator),125I-labeled albumin and [14C] sucrose (interstitual indicators), and [3H]2O (whole tissue indicator) into the portal vein at a perfusion rate of 12 ml/min. The outflow profiles based on the DM were convolved with the transport function of the catheters, then fitted to the data. A fairly good fit was obtained for most of the MID curve, with the exception of the late-in-time data (prolonged tail) beyond \(3 \times \overline {\text{t}}\). The fitted DNs were found to differ among the indicators, and not with the length of the inflow and outflow catheters. But the differences disappeared when a delay parameter, t0=4.1 ± 0.7 sec \(\left( {\overline x \pm SD} \right)\), was included as an additional fitted parameter for all of the indicators except water. Using the short catheters, the average DNfor the model with delay was 0.31 ± 0.13 for closed and 0.22 ± 0.07 for mixed boundary conditions, for all reference indicators. Mean transit times and the variances of the fitted distributions were always smaller than the experimental ones (on average, by 6.8 ± 3.7% and 58 ± 19%, respectively). In conclusion, the DM is a reasonable descriptor of dispersion for the early-in-time data and not the late-in-time data. The existence of a common DN for all non-eliminated reference indicators suggests that intrahepatic dispersion depends only on the geometry of the vasculature rather than the diffusional processes. The role of the nonsinusoidal (“large”) vessels can be partly represented by a simple delay.
Similar content being viewed by others
REFERENCES
R. S. McCuskey. The hepatic microvascular system. In I. M. Arias (ed.), The Liver, Raven Press, New York, 1994, pp. 1089–1106.
A. Koo, I. Y. Liang, and K. K. Cheng. The terminal hepatic microcirculation in the rat. Quant. J. Exp. Physiol. Cognate Med. Sci. 60:261–266 (1975).
C. A. Goresky. A linear method for determining liver sinusoidal and extravascular volumes. Am. J. Physiol. 204:626–640 (1963).
C. A. Goresky, W. H. Ziegler, and G. G. Bach. Capillary exchange modeling. Barrier-limited and flow-limited distribution. Circ. Res. 27:739–764 (1970).
C. A. Goresky. Kinetic interpretation of hepatic multiple-indicator dilution studies. Am. J. Physiol. 245:G1–G12 (1983).
S. S. Kety. The measurement of regional circulation by local clearance of radioactive sodium. Am. Heart J. 38:321–328 (1949).
K. Winkler, S. Keiding, and N. Tygstrup. Clearance as a quantitative measure of liver function. In R. P. P. Paumgartner (ed.), The Liver: Quantitative Aspects of Structure and Function, S. Karger, Basel, 1973, pp. 144–155.
A. B. Ahmad, P. N. Bennett, and M. Rowland. Influence of route of hepatic administration on drug availability. J. Pharmacol. Exp. Ther. 230:718–725 (1984).
A. B. Ahmad, P. N. Bennett, and M. Rowland. Models of hepatic drug clearance: Discrimination between the “well stirred” and “parallel-tube” models. J. Pharm. Pharmacol. 35:219–224 (1983).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J. Pharmacokin. Biopharm. 5:625–653 (1977).
D. L. Miller, C. S. Zanolli, and J. J. Gumucio. Quantitative morphology of the sinusoids of the hepatic acinus. Quantimet analysis of rat liver. Gastroenterology 76:965–969 (1979).
M. S. Roberts and M. Rowland. Hepatic elimination-dispersion model. J. Pharm. Sci. 74:585–587 (1985).
M. S. Roberts and M. Rowland. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations. J. Pharmacokin. Biopharm. 14:227–260 (1986).
M. Weiss. A note on the interpretation of tracer dispersion in the liver. J. Theoret. Biol. 184:1–6 (1997).
R. Tirona, A. J. Schwab, W. Geng, and K. S. Pang. Comparison of the Dispersion and Goresky models in outflow dilution profiles from multiple indicator rat liver studies. Drug Metab. Dispos. 26:465–475 (1998).
P. V. Danckwerts. Continuous flow systems: distribution of residence times. Chem. Eng. Sci. 2:1–13 (1953).
G. I. Taylor. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. Roy. Soc. Lond. Ser. A 219:186–203 (1953).
G. I. Taylor. The dispersion of matter in turbulent flow through a pipe. Proc. Roy. Soc. Lond. Ser. A 223:446–468 (1954).
A Griffiths. On the movement of a coloured index along a capillary tube, and its application to the measurement of the circulation of water in a closed circuit. Proc. Phys. Soc. 23:190–197 (1911).
W. Perl and F. P. Chinard. A convection-diffusion model of indicator transport through an organ. Circ. Res. 22:273–298 (1968).
C. W. Sheppard and L. J. Savage. The random walk problem in relation to the physiology of circulatory mixing. Phys. Rev. 83:489–490 (1951).
Z.-S. Cai, B. A. Luxon, and E. L. Forker. Intralobular zonal heterogeneity and hepatic indicator dilution curves. Am. J. Physiol. 268:G189–G199 (1995).
S. H. Audi, C. A. Dawson, J. H. Linehan, G. S. Krenz, S. B. Ahlf, and D. L. Roerig. An interpretation of 14C-urea and 14C-primidone extraction in isolated rabbit lungs. Ann. Biomed. Eng. 24:337–351 (1996).
C. A. Goresky and M. Silverman. Effect of the correction of catheter distortion on calculated liver sinusoidal volumes. Am. J. Physiol. 207:883–892 (1964).
A. M. Evans, Z. Hussein, and M. Rowland. Influence of albumin on the distribution and elimination kinetics of diclofenac in the isolated perfused rat liver: analysis by the impulse-response technique and the dispersion model. J. Pharm. Sci. 82:421–428 (1993).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. II. Experimental evidence for acceptance of the “well-stirred” model over the “parallel tube” model using lidocaine in the perfused rat liver in situ preparation. J. Pharmacokin. Biopharm. 5:655–680 (1977).
K. S. Pang and J. A. Terrell. Retrograde perfusion to probe the heterogeneous distribution of hepatic drug metabolizing enzymes in rats. J. Pharmacol. Exp. Ther. 216:339–346 (1981).
W. P. Geng, A. J. Schwab, C. A. Goresky, and K. S. Pang. Carrier-mediated uptake and excretion of bromosulfophthalein-glutathione in perfused rat liver: a multiple indicator dilution study. Hepatology 22:1188–1207 (1995).
N. Xu, A. Chow, C. A. Goresky, and K. S. Pang. Effects of retrograde flow on measured blood volume, Disse space, intracellular water space and drug extraction in the perfused rat liver: characterization by the multiple indicator dilution technique. J. Pharmacol. Exp. Ther. 254:914–925 (1990).
M. V. St-Pierre, A. J. Schwab, C. A. Goresky, W. F. Lee, and K. S. Pang. The multiple-indicator dilution technique for characterization of normal and retrograde flow in once-through rat liver perfusions. Hepatology 9:285–296 (1989).
K. S. Pang, W. F. Lee, W. F. Cherry, V. Yuen, J. Accaputo, S. Fayz, A. J. Schwab, and C. A. Goresky. Effects of perfusate flow rate on measured blood volume, Disse space, intracellular water space, and drug extraction in the perfused rat liver preparation: characterization by the multiple indicator dilution technique. J. Pharmacokin. Biopharm. 16:595–632 (1988).
K. S. Pang, F. Barker, III, A. J. Schwab, and C. A. Goresky. Demonstration of rapid entry and a cellular binding space for salicylamide in perfused rat liver: a multiple indicator dilution study. J. Pharmacol. Exp. Ther. 270:285–295 (1994).
M. V. St-Pierre, P. I. Lee, and K. S. Pang. A comparative investigation of hepatic clearance models: predictions of metabolite formation and elimination. J. Pharmacokin. Biopharm. 20:105–145 (1992).
J. B. Bassingthwaighte. A concurrent flow model for extraction during transcapillary passage. Circ. Res. 35:483–503 (1974).
J. B. Bassingthwaighte, I. S. Chan, and C. Y. Wang. Computationally efficient algorithms for convection-permeation-diffusion models for blood-tissue exchange. Ann. Biomed. Eng. 20:687–725 (1992).
L. P. Rivory, M. S. Roberts, and S. M. Pond. Axial tissue diffusion can account for the disparity between current models of hepatic elimination for lipophilic drugs. J. Pharmacokin. Biopharm. 20:19–61 (1992).
B. A. Luxon and R. A. Weisiger. Extending the multiple indicator dilution method to include slow intracellular diffusion. Math. Biosci. 113:211–230 (1993).
B. A. Luxon and R. A. Weisiger. A new method for quantitating intracellular transport: application to the thyroid hormone 3,5,3′-triiodothyronine. Am. J. Physiol. 263:G733–G741 (1992).
C. Sheppard. Basic Principles of the Tracer Method, Wiley, New York, 1962.
T. R. Harris and E. V. Newman. An analysis of mathematical models of circulatory indicator-dilution curves. J. Appl. Physiol. 28:840–850 (1970).
A. J. Schwab. Extension of the theory of the multiple-indicator dilution technique to metabolic systems with an arbitrary number of rate constants. Math. Biosci. 71:57–79 (1984).
M. R. Gray and Y. K. Tam. The series-compartment model for hepatic elimination. Drug. Metab. Dispos. 15:27–31 (1987).
C. Cobelli, M. P. Saccomani, E. Ferannini, R. A. Defronzo, R. Gelfand, and R. Bonadonna. A compartmental model to quantitate in vivo glucose transport in the human forearm. Am. J. Physiol. 257:E943–E958 (1989).
M. E. Wise. Tracer dilution curves in cardiology and random walk and lognormal distributions. Acta Physiol. Pharmacol. Neerl. 14:175–204 (1966).
J. B. Bassingthwaighte, F. H. Ackerman, and E. H. Wood. Applications of the lagged normal density curve as a model for arterial dilution curves. Circ. Res. 18:398–415 (1966).
C. P. Rose, C. A. Goresky, P. Belanger, and M. J. Chen. Effect of vasodilation and flow rate on capillary permeability surface product and interstitial space size in the coronary circulation. A frequency domain technique for modeling multiple dilution data with Laguerre functions. Circ. Res. 47:312–328 (1980).
S. E. Salcudean, P. R. Belanger, C. A. Goresky, and C. P. Rose. The use of Laguerre functions for parameter identification in a distributed biological system. IEEE Trans. Biomed. Eng. 28:767–775 (1981).
H. Thompson, C. Starmer, R. Whalen, and H. MacIntosh. Indicator transit time considered as a gamma variate. Circ. Res. 14:502–515 (1964).
W. Weiss. Use of gamma distributed residence times in pharmacokinetics. Eur. J. Clin. Pharmacol. 25:695–702 (1983).
R. Rowlett and T. Harris. A comparative study of organ models and numerical techniques for the evaluation of capillary permeability from multiple-indicator data. Math. Biosci. 29:273–298 (1976).
M. S. Roberts, J. D. Donaldson, and M. Rowland. Models of hepatic elimination: comparison of stochastic models to describe residence time distributions and to predict the influence of drug distribution, enzyme heterogeneity, and systemic recycling on hepatic elimination. J. Pharmacokin. Biopharm. 16:41–83 (1988).
M. S. Roberts and M. Rowland. A dispersion model of hepatic elimination: 2. Steady-state considerations—influence of hepatic blood flow, binding within blood, and hepatocellular enzyme activity. J. Pharmacokin. Biopharm. 14:261–288 (1986).
M. S. Roberts and M. Rowland. A dispersion model of hepatic elimination: 3. Application to metabolite formation and elimination kinetics. J. Pharmacokin. Biopharm. 14:289–308 (1986).
K. Ueda, K. Yamaoka, M. E. Rodriguez, A. Shibukawa, and T. Nakagawa. Enantioselective local disposition of semotiadil (R-enantiomer) and levosemotiadil (S-enantiomer) in perfused rat liver. Drug Metab. Dispos. 25:281–286 (1997).
F. J. Burczynski, B. A. Luxon, and R. A. Weisiger. Intrahepatic blood flow distribution in the perfused rat liver: effect of hepatic artery perfusion. Am. J. Physiol. 271:G561–G567 (1996).
C. A. Goresky, A. J. Schwab, and C. P. Rose. Xenon handling in the liver: red cell capacity effect. Circ. Res. 63:767–778 (1988).
R. B. King, A. Deussen, G. M. Raymond, and J. B. Bassingthwaighte. A vascular transport operator. Am. J. Physiol. 265:H2196–H2208 (1993).
Y. Yano, K. Yamaoka, Y. Aoyama, and H. Tanaka. Two-compartment dispersion model for analysis of organ perfusion system of drugs by fast inverse Laplace transform (FILT). J. Pharmacokin. Biopharm. 17:179–202 (1989).
J. M. Diaz-García, A. M. Evans, and M. Rowland. Application of the axial dispersion model of hepatic drug elimination to the kinetics of diazepam in the isolated perfused rat liver. J. Pharmacokin. Biopharm. 20:171–193 (1992).
M. S. Roberts, S. Frazer, A. Wagner, and L. McLeod. Residence time distributions of solutes in the perfused rat liver using a dispersion model of hepatic elimination: 1. Effect of changes in perfusate flow and albumin concentration on sucrose and taurocholate. J. Pharmacokin. Biopharm. 18:209–234 (1990).
H. Yasui, K. Yamaoka, T. Fukuyama, and T. Nakagawa. Effect of liver intoxication by carbon tetrachloride on hepatic local disposition of oxacillin using moment characteristics as index. Drug Metab. Dispos. 23:779–785 (1995).
Y. Ohata, K. Yamaoka, H. Yasui, and T. Nakagawa. Consideration on moments of outflow profile in liver perfusion system with change in perfusate flow rate using oxacillin as model drug. Biol. Pharm. Bull. 19:83–87 (1996).
M. S. Roberts, S. Fraser, A. Wagner, and L. McLeod. Residence time distributions of solutes in the perfused rat liver using a dispersion model of hepatic elimination: 2. Effect of pharmacological agents, retrograde perfusions, and enzyme inhibition on Evans blue, sucrose, water, and taurocholate. J. Pharmacokin. Biopharm. 18:235–258 (1990).
R. H. Smallwood, D. J. Morgan, G. W. Mihaly, D. B. Jones, and R. A. Smallwood. Effect of plasma protein binding on elimination of taurocholate by isolated perfused rat liver: comparison of venous equilibrium, undistributed and distributed sinusoidal, and dispersion models. J. Pharmacokin. Biopharm. 16:377–396 (1988).
M. S. Ching, D. J. Morgan, and R. A. Smallwood. Models of hepatic elimination: Implications from studies of the simultaneous elimination of taurocholate and diazepam by isolated rat liver under varying conditions of binding. J. Pharmacol. Exp. Ther. 250:1048–1054 (1989).
Z. Hussein, A. M. Evans, and M. Rowland. Physiologic models of hepatic drug clearance: influence of altered protein binding on the elimination of diclofenac in the isolated perfused rat liver. J. Pharm. Sci. 82:880–885 (1993).
L. Bass. Convection-dispersion modeling of hepatic elimination. J. Pharm. Sci. 75:321–322 (1986).
C. A. Goresky, G. C. Bach, and B. E. Nadeau. On the uptake of materials by the intact liver. The concentrative transport of rubidium-86. J. Clin. Invest. 52:975–990 (1973).
J. Kuikka, M. Levin, and J. B. Bassingthwaighte. Multiple tracer dilution estimates of D-and 2-deoxy-D-glucose uptake by the heart. Am. J. Physiol. 250:H29–H42 (1986).
R. B. King, G. M. Raymond, and J. B. Bassingthwaighte. Modeling blood flow heterogeneity. Ann. Biomed. Eng. 24:352–372 (1996).
J. B. Bassingthwaighte and D. A. Beard. Fractal 15O-labeled water washout from the heart. Circ. Res. 77:1212–1221 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwab, A.J., Geng, W. & Pang, K.S. Application of the Dispersion Model for Description of the Outflow Dilution Profiles of Noneliminated Reference Indicators in Rat Liver Perfusion Studies. J Pharmacokinet Pharmacodyn 26, 163–181 (1998). https://doi.org/10.1023/A:1020557706994
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1020557706994