Abstract
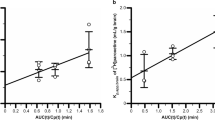
Purpose. To determine concentration-dependent P-gp-mediated efflux across the luminal membrane of endothelial cells at the blood-brain barrier (BBB) in rats.
Methods. The transport of radiolabeled colchicine and vinblastine across the rat BBB was measured with or without PSC833, a well known P-gp inhibitor, and within a wide range of colchicine and vinblastine concentration by an in situ brain perfusion. Thus, the difference of brain transport achieved with or without PSC833 gives the P-gp-mediated efflux component of the compound transported through the rat BBB. Cerebral vascular volume was determined by coperfusion with labeled sucrose in all experiments.
Results. Sucrose perfusion indicated that the vascular space was close to normal in all the studies, indicating that the BBB remained intact. P-gp limited the uptake of both colchicine and vinblastine, but the compounds differ in that vinblastine inhibited its own transport. Vinblastine transport was well fitted by a Hill equation giving IC50 at ∼71 μM, a Hill coefficient (n) ∼2, and a maximal efflux velocity Jmax of ∼9 pmol s−1 g−1 of brain.
Conclusions. P-gp at the rat BBB may carry out both capacity-limited and capacity-unlimited transport, depending on the substrate, with pharmacotoxicologic significance for drug brain disposition and risk of drug-drug interactions.
Similar content being viewed by others
References
C. Cordon-Cardo, J. P. O'Brien, D. Casals, L. Rittman-Grauer, J. L. Biedler, M. R. Melamed, and R. Bertino. Multidrug resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 86:695-698 (1989).
Y. Takasato, S. I. Rapoport, and Q. R. Smith. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 247:H484-H493 (1984).
Q. R. Smith. Brain perfusion systems for studies of drug uptake and metabolism in the central nervous system. In: R. T. Borchardt, P. L. Smith, G. Wilson (eds.), Models for Assessing Drug Absorption and Metabolism, Vol. 8. Plenum Press, New York, 1996, pp. 285-307.
S. Cisternino, C. Rousselle, C. Dagenais, and J. M. Scherrmann. Screening of multidrug-resistance sensitive drugs by in situ brain perfusion in P-glycoprotein-deficient mice. Pharm. Res. 18:183-190 (2001).
A. H. Schinkel, E. Wagenaar, L. van Deemter, C. A. Mol, and P. Borst. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 96:1698-1705 (1995).
C. H. Rousselle, J. M. Lefauconnier, and D. D. Allen. Evaluation of anesthetic effects on parameters for the in situ rat brain perfusion technique. Neurosci. Lett. 257:139-142 (1998).
C. Martin, G. Berridge, C. F. Higgins, P. Mistry, P. Charlton, and R. Callaghan. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 58:624-632 (2000).
J. C. Taylor, D. R. Ferry, C. F. Higgins, and R. Callaghan. The equilibrium and kinetic drug binding properties of the mouse P-gp1a and P-gp1b P-glycoproteins are similar. Br. J. Cancer 81:783-789 (1999).
A. M. Taylor, J. Storm, L. Soceneantu, K. J. Linton, M. Gabriel, C. Martin, J. Woodhouse, E. Blott, C. F. Higgins, and R. Callaghan. Detailed characterization of cysteine-less P-glycoprotein reveals subtle pharmacological differences in function from wild-type protein. Br. J. Pharmacol. 134:1609-1618 (2001).
R. Liu and F. J. Sharom. Site-directed fluorescence labeling of P-glycoprotein on cysteine residues in the nucleotide binding domains. Biochemistry 35:11865-11873 (1996).
T. E. Druley, W. D. Stein, A. Ruth, and I. B. Roninson. P-glycoprotein-mediated colchicine resistance in different cell lines correlates with the effects of colchicine on P-glycoprotein conformation. Biochemistry 40:4323-4331 (2001).
E. J. Wang, C. N. Casciano, R. P. Clement, and W. W. Johnson. Cooperativity in the inhibition of P-glycoprotein-mediated daunorubicin transport: evidence for half-of-the-sites reactivity. Arch. Biochem. Biophys. 383:91-98 (2000).
R. H. Stephens, C. A. O'Neill, A. Warhurst, G. L. Carlson, M. Rowland, and G. Warhurst. Kinetic profiling of P-glycoprotein-mediated drug efflux in rat and human intestinal epithelia. J. Pharmacol. Exp. Ther. 296:584-591 (2001).
M. J. Borgnia, G. D. Eytan, and Y. G. Assaraf. Competition of hydrophobic peptides, cytotoxic drugs, and chemosensitizers on a common P-glycoprotein pharmacophore as revealed by its ATPase activity. J. Biol. Chem. 271:3163-3171 (1996).
I. L. Urbatsch and A. E. Senior. Effects of lipids on ATPase activity of purified Chinese hamster P-glycoprotein. Arch. Biochem. Biophys. 316:135-140 (1995).
S. Dey, P. Hafkemeyer, I. Pastan, and M. M. Gottesman. A single amino acid residue contributes to distinct mechanisms of inhibition of the human multidrug transporter by stereoisomers of the dopamine receptor antagonist flupenthixol. Biochemistry 38:6630-6639 (1999).
M. M. Gottesman, I. Pastan, and S. V. Ambudkar. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 6:610-617 (1996).
Z. E. Sauna, M. M. Smith, M. Muller, K. M. Kerr, and S. V. Ambudkar. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J. Bioenerg. Biomembr. 33:481-491 (2001).
P. Lu, R. Liu, and F. J. Sharom. Drug transport by reconstituted P-glycoportein in proteoliposomes. Effect of substrates and modulators, and dependence on bilayer phase state. Eur. J. Biochem. 268:1687-1697 (2001).
M. F. Fromm, R. B. Kim, C. M. Stein, G. R. Wilkinson, and D. M. Roden. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine. Circulation 99:552-557 (1999).
Y. Adachi, H. Suzuki, and Y. Sugiyama. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm. Res. 18:1660-1668 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cisternino, S., Rousselle, C., Debray, M. et al. In Vivo Saturation of the Transport of Vinblastine and Colchicine by P-Glycoprotein at the Rat Blood–Brain Barrier. Pharm Res 20, 1607–1611 (2003). https://doi.org/10.1023/A:1026187301648
Issue Date:
DOI: https://doi.org/10.1023/A:1026187301648