Abstract
Purpose. The aim was to replace the traditional 21-day Caco-2 permeability protocol by a more high-throughput assay.
Methods. Caco-2 cells were seeded at high density in 96-well plates in novel cell culture boxes. After 7 days, drug permeability studies were performed. Samples were analyzed by a new UV detection method.
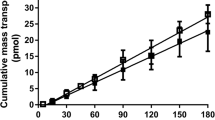
Results. With increased cell seeding density, functional Caco-2 monolayers with polarized efflux transporters were established after 7 days in 96-well polycarbonate filter plates in standard medium. For faster feeding and to eliminate medium replacement in each individual well, plates were completely submerged in medium in novel cell culture boxes, and only medium outside the plate was exchanged. For high-throughput sample analysis, a novel UV-transparent transport buffer was established that allowed direct quantification of permeated drug from its UV absorption. In vitro permeability studies analyzing 22 passively absorbed drugs in the new model correlated well with reported human permeability values (r2 = 0.8725).
Conclusions. The new 7-day, 96-well Caco-2 permeability model tight to UV analysis offers considerable time, cost, and resource savings compared to the traditional model. It has a potential for automation and makes it possible to determine the permeability of passively diffusing compounds and to classify them according to the BCS in a truly medium- to high-throughput mode.
Similar content being viewed by others
REFERENCES
P. Artursson and J. Karlsson. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochim. Biophys. Res. Commun 175:880-885 (1991).
T. Mainprize and L. Grady. Standardization of an in vitro method of drug absorption. Pharmacopeial Forum 24:6015-6023 (1998).
P. Artursson, K. Palm, and K. Luthman. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46:27-43 (2001).
G. L. Amidon, H. Lennernäs, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413-420 (1995).
S. Chong, S. Dando, and R. Morrison. Evaluation of Biocoat intestinal epithelium differentiation environment (3-day cultured Caco-2 cells) as an absorption screening model with improved productivity. Pharm. Res. 14:1835-1837 (1997).
E. Liang, K. Chessic, and M. Yazdanian. Evaluation of an accelerated Caco-2 cell permeability model. J. Pharm. Sci. 89:336-345 (2000).
J. Irvine, L. Takahashi, K. Lockhard, J. Cheong, J. Tolan, H. Selick, and J. Grove. MDCK (Madin-Darby Canine Kidney) cells: a tool for membrane permeability screening. J. Pharm. Sci. 88:28-33 (1999).
P. Garberg, P. Eriksson, N. Schipper, and B. Sjöström. Automated absorption assessment using Caco-2 cells cultured on both sides of polycarbonate membranes. Pharm. Res. 16:441-445 (1999).
H. Bu, M. Poglod, and R. Micetich, and J. Khan. High-throughput Caco-2 cell permeability screening by cassette dosing and sample pooling approaches using direct injection/on-line guard cartridge extraction/tandem mass spectrometry. Rapid Commun. Mass Spectrum 14:523-528 (2000).
W. Taylor, J. Gibbons, and R. Braeckman. Intestinal absorption screening of mixtures from combinatorial libraries in the Caco-2 model. Pharm. Res. 14:572-577 (1997).
M. Correll, S. Broward-Partin, D. Hansen, H. Behlow, M. Lewis, J. Rose, and T. Thompson. Achievement of higher throughput in the Caco-2 absorption screen using a simplified experimental design. AAPS Annual Meeting October 25-28, 2000, St. Louis, MO.
K. Lentz, J. Hayashi, L. Lucisano, and J. E. Polli. Development of a more rapid, reduced serum culture system for Caco-2 monolayers and application to the biopharmaceutics classification system. Int. J. Pharm. 200:41-51 (2000).
H. Yu, K. Laws, M. Rooney, N. Spicer, V. Doan, O. Chan, and B. Janosky. Validation of the 96-well transport plate in the Caco-2 permeability screening assay. AAPS Annual Meeting November 10-14, 2000, Toronto, Canada
J. Alsenz, H. Steffen, and R. Alex. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm. Res. 15:423-428 (1998).
S. Yamashita, K. Konishi, Y. Yamazaki, Y. Taki, T. Sakane, H. Sezaki, and Y. Furuyama. New and better protocols for a short-term Caco-2 cell culture system. J. Pharm. Sci. 91:669-679 (2002).
H. Lennernäs, K. Gjellan, R. Hällgren, and C. Graffner. The influence of caprate on rectal absorption of phenoxymethylpenicillin: experience from an in-vivo perfusion in humans. J. Pharm. Pharmacol. 54:499-508 (2002).
H. Lennernäs, L. Knutson, A. Hussain, L. Lesko, T. Salmonson, and G. Amidon. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: experience from a regional perfusion technique. Eur. J. Pharm. Sci. 15:271-277 (2002).
Z. Wang, C. Hop, K. Leumg, and J. Pang. Determination of in vitro permeability of drug candidates through a Caco-2 cell monolayer by a liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 35:71-76 (2000).
M. Markowska, R. Oberle, S. Juzwin, C. Hsu, M. Gryszkiewicz, and A. Streeter. Optimizing Caco-2 monolayers to increase throughput in drug intestinal absorption analysis. J. Pharmacol. Toxicol. Meth. 46:51-55 (2001).
K. Bogman, F. Erne-Brand, J. Alsenz, and J. Drewe. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J. Pharm. Sci. 92:1250-1261 (2003).
L. Jorgensen, P. Artursson, and E. Bechgaard. Toxicological and absorption enhancing effects of glycofurol 75 and sodium glycocholate in monolayers of human intestinal epithelial (Caco-2) cells. Int. J. Pharm. 95:209-217 (1993).
R. Froquet, Y. Sibiril, and D. Parent-Massin. Improvement of megakaryocytic progenitor culture for toxicological investigations. Toxicology in Vitro 15:691-699 (2001).
D. Sun, H. Lennernäs, L. Welage, J. Barnett, C. Landowski, D. Foster, D. Fleisher, K. Lee, and G. Amidon. Comparison of human duodenum and Caco-2 gene expression profiles for 12000 gene sequence tags and correlation with permeability of 26 drugs. Pharm. Res. 19:1400-1416 (2002).
S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, and H. Tokuda. Optimized conditions for the prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 10:195-204 (2000).
S. Winiwarter, N. M. Bonham, F. Ax, A. Hallberg, H. Lennernäs, and A. Karlén. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariant analysis approach. J. Med. Chem. 41:4939-4949 (1998).
J. Dressman, J. Butler, J. Hempenstall, and C. Reppas. The BCS: Where do we go from here? Pharm. Tech. July:68-76 (2001).
C. Bergström, M. Strafford, L. Lazorova, A. Avdeef, K. Luthman, and P. Artursson. Absorption classification of oral drugs based on molecular surface properties. J. Med. Chem. 46:558-570 (2003).
N. Takamatsu, O.-N. Kim, L. S. Welage, N. M. Idkaidek, Y. Hayashi, J. Barnett, R. Yamamoto, E. Lipka, H. Lennernäs, A. Hussain, L. Lesko, and G. L. Amidon. Human jejunal permeability of two polar drugs: cimetidine and ranitidine. Pharm. Res. 18:742-744 (2001).
G. Amidon. (1996). Second International Workshop on Strategies for Oral Drug Delivery, Ascona, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alsenz, J., Haenel, E. Development of a 7-Day, 96-Well Caco-2 Permeability Assay with High-Throughput Direct UV Compound Analysis. Pharm Res 20, 1961–1969 (2003). https://doi.org/10.1023/B:PHAM.0000008043.71001.43
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000008043.71001.43