Abstract
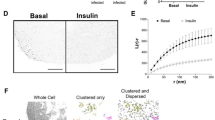
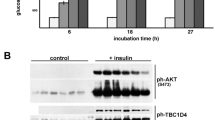
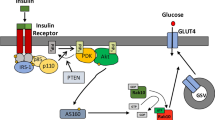
Insulin stimulates glucose uptake in fat and muscle by mobilizing the GLUT4 glucose transporter. GLUT4 is sequestered intracellularly in the absence of insulin, and is redistributed to the plasma membrane within minutes of insulin stimulation1,2. But the trafficking mechanisms that control GLUT4 sequestration have remained elusive. Here we describe a functional screen to identify proteins that modulate GLUT4 distribution, and identify TUG as a putative tether, containing a UBX domain, for GLUT4. In truncated form, TUG acts in a dominant-negative manner to inhibit insulin-stimulated GLUT4 redistribution in Chinese hamster ovary cells and 3T3-L1 adipocytes. Full-length TUG forms a complex specifically with GLUT4; in 3T3-L1 adipocytes, this complex is present in unstimulated cells and is largely disassembled by insulin. Endogenous TUG is localized with the insulin-mobilizable pool of GLUT4 in unstimulated 3T3-L1 adipocytes, and is not mobilized to the plasma membrane by insulin. Distinct regions of TUG are required to bind GLUT4 and to retain GLUT4 intracellularly in transfected, non-adipose cells. Our data suggest that TUG traps endocytosed GLUT4 and tethers it intracellularly, and that insulin mobilizes this pool of retained GLUT4 by releasing this tether.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bryant, N. J., Govers, R. & James, D. E. Regulated transport of the glucose transporter GLUT4. Nature Rev. Mol. Cell Biol. 3, 267–277 (2002)
Holman, G. D. & Sandoval, I. V. Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol. 11, 173–179 (2001)
Bogan, J. S., McKee, A. E. & Lodish, H. F. Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol. Cell. Biol. 21, 4785–4806 (2001)
Liu, X. et al. Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal. Biochem. 280, 20–28 (2000)
Kondo, H. et al. p47 is a cofactor for p97-mediated membrane fusion. Nature 388, 75–78 (1997)
Chu, K., Niu, X. & Williams, L. T. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc. Natl Acad. Sci. USA 92, 11894–11898 (1995)
Buchberger, A., Howard, M. J., Proctor, M. & Bycroft, M. The UBX domain: a widespread ubiquitin-like module. J. Mol. Biol. 307, 17–24 (2001)
Yuan, X. et al. Solution structure and interaction surface of the C-terminal domain from p47: a major p97-cofactor involved in SNARE disassembly. J. Mol. Biol. 311, 255–263 (2001)
Ladanyi, M. et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20, 48–57 (2001)
Giorgino, F. et al. The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc. Natl Acad. Sci. USA 97, 1125–1130 (2000)
Becker, K., Schneider, P., Hofmann, K., Mattmann, C. & Tschopp, J. Interaction of Fas(Apo-1/CD95) with proteins implicated in the ubiquitination pathway. FEBS Lett. 412, 102–106 (1997)
Wright, D. A., Futcher, B., Ghosh, P. & Geha, R. S. Association of human fas (CD95) with a ubiquitin-conjugating enzyme (UBC-FAP). J. Biol. Chem. 271, 31037–31043 (1996)
Lalioti, V. S., Vergarajauregui, S., Pulido, D. & Sandoval, I. V. The insulin-sensitive glucose transporter, GLUT4, interacts physically with Daxx. Two proteins with capacity to bind Ubc9 and conjugated to SUMO-1. J. Biol. Chem. 277, 19783–19791 (2002)
Shepherd, P. R. & Kahn, B. B. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341, 248–257 (1999)
Perera, H. K. I. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell 14, 2946–2958 (2003)
Katome, T. Use of RNA-interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J. Biol. Chem. 278, 28312–28323 (2003)
Inoue, M., Chang, L., Hwang, J., Chiang, S. H. & Saltiel, A. R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629–633 (2003)
Bose, A. et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821–824 (2002)
Wilson, V. G. & Rangasamy, D. Intracellular targeting of proteins by sumoylation. Exp. Cell Res. 271, 57–65 (2001)
Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70, 5701–5705 (1996)
Tordjman, K. M., Leingang, K. A., James, D. E. & Mueckler, M. M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: effect of chronic insulin and tolbutamide treatment. Proc. Natl Acad. Sci. USA 86, 7761–7765 (1989)
Bogan, J. S. & Lodish, H. F. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J. Cell Biol. 146, 609–620 (1999)
Acknowledgements
We thank P. Bickel for help with cDNA library construction, G. Paradis for assistance with flow cytometry, J. Cresswell for technical assistance, and N.-W. Chi, J. Avruch, J. Dziura and K. Calia for advice and encouragement. We thank I. Verma for the pCL-Eco plasmid, G. Nolan for retroviral packaging cell lines, M. Charron for GLUT4 antisera, M. Krieger for CHO cells expressing the ecotropic murine retrovirus receptor, and N. Watson and S. Zarnegar for help with microscopy. This work used the W.M. Keck Foundation Biological Imaging Facility at the Whitehead Institute, and the Center for Cell Imaging at Yale University. This work was supported by a New Investigator Award from the Chestnut Hill Charitable Foundation to J.S.B., by a Career Development Award from the American Diabetes Association to J.S.B., by a Pilot and Feasibility Award from the NIH-sponsored Boston Area Diabetes Endocrinology Research Center to J.S.B., by a postdoctoral fellowship from the Ares-Serono Foundation to T.-S.T., and by NIH grants to H.F.L. and J.S.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Bogan, J., Hendon, N., McKee, A. et al. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425, 727–733 (2003). https://doi.org/10.1038/nature01989
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01989
This article is cited by
-
Exploring the role of ubiquitin regulatory X domain family proteins in cancers: bioinformatics insights, mechanisms, and implications for therapy
Journal of Translational Medicine (2024)
-
ASPSCR1-TFE3 reprograms transcription by organizing enhancer loops around hexameric VCP/p97
Nature Communications (2024)
-
Inhibitors of RNA and protein synthesis cause Glut4 translocation and increase glucose uptake in adipocytes
Scientific Reports (2022)
-
Insulin-stimulated endoproteolytic TUG cleavage links energy expenditure with glucose uptake
Nature Metabolism (2021)
-
Identification of strong candidate genes for backfat and intramuscular fatty acid composition in three crosses based on the Iberian pig
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.