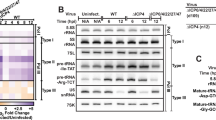
Abstract
Small interfering RNAs (siRNA) are potent reagents for directed post-transcriptional gene silencing1 and a major new genetic tool for investigating mammalian cells. When synthetic siRNAs are used for gene silencing, the costs can be substantial because of variations in siRNA efficacies. An alternative to chemically synthesized siRNAs are siRNAs produced by bacteriophage T7 RNA polymerase. We found that siRNAs synthesized from the T7 RNA polymerase system can trigger a potent induction of interferon α and β in a variety of cell lines. Surprisingly, we also found very potent induction of interferon α and β by short single-stranded RNAs (ssRNAs) transcribed with T3, T7 and Sp6 RNA polymerases. Analyses of the potential mediators of this response revealed that the initiating 5′ triphosphate is required for interferon induction. We describe here an improved method for T7 siRNA synthesis that alleviates the interferon response while maintaining full efficacy of the siRNAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hannon, G.J. RNA interference. Nature 418, 244–251 (2002).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Andino, R. RNAi puts a lid on virus replication. Nat. Biotechnol. 21, 629–630 (2003).
Sohail, M., Doran, G., Riedemann, J., Macaulay, V. & Southern, E.M. A simple and cost-effective method for producing small interfering RNAs with high efficacy. Nucleic Acids Res. 31, e38 (2003).
Donze, O. & Picard, D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res. 30, e46 (2002).
Elliott, G. & O'Hare, P. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J. Virol. 73, 4110–4119 (1999).
Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 14, 778–809 (2001).
Stojdl, D.F. et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6, 821–825 (2000).
Kim, D.H. & Rossi, J.J. Coupling of RNAi-mediated target downregulation with gene replacement. Antisense Nucleic Acid Drug Dev. 13, 151–155 (2003).
Wang, L. et al. Mapping oligonucleotides of Rous sarcoma virus RNA that segregate with polymerase and group-specific antigen markers in recombinants. Proc. Natl. Acad. Sci. USA 73, 3952–3956 (1976).
Capodici, J., Kariko, K. & Weissman, D. Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J. Immunol. 169, 5196–5201 (2002).
Kapadia, S.B., Brideau-Andersen, A. & Chisari, F.V. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc. Natl. Acad. Sci. USA 100, 2014–2018 (2003).
Sledz, C.A., Holko, M., de Veer, M.J., Silverman, R.H. & Williams, B.R. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–839 (2003).
Honda, A., Mizumoto, K. & Ishihama, A. Identification of the 5′ terminal structure of influenza virus genome RNA by a newly developed enzymatic method. Virus Res. 55, 199–206 (1998).
Acknowledgements
This work was supported by a grant from the Arnold and Mabel Beckman Foundation and the National Institutes of Health (AI29329, AI42552 and HL074704 to J.J.R.). D. Kim is a Beckman Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kim, DH., Longo, M., Han, Y. et al. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat Biotechnol 22, 321–325 (2004). https://doi.org/10.1038/nbt940
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt940
This article is cited by
-
Innate immune regulations and various siRNA modalities
Drug Delivery and Translational Research (2023)
-
In vitro transcribed sgRNA causes cell death by inducing interferon release
Protein & Cell (2019)
-
Novel RNA Duplex Locks HIV-1 in a Latent State via Chromatin-mediated Transcriptional Silencing
Molecular Therapy - Nucleic Acids (2015)
-
Overexpression of SIRT1 Induced by Resveratrol and Inhibitor of miR-204 Suppresses Activation and Proliferation of Microglia
Journal of Molecular Neuroscience (2015)
-
Therapeutic potential of siRNA and DNAzymes in cancer
Tumor Biology (2014)