Abstract
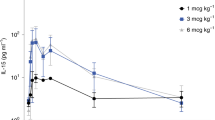
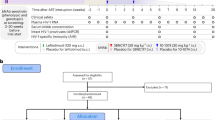
We assessed the efficacy and safety of 10-d monotherapy with the orally administered CCR5 antagonist maraviroc in 63 HIV-1-positive individuals prescreened for the absence of CXCR4-using virus. Maximum reduction in viral load occurred at a median of 10–15 d, with a mean reduction of ≥1.6 log10 copies/ml at all twice daily doses ≥100 mg. These results provide proof of concept that CCR5 antagonism is a viable antiretroviral therapeutic approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Struble, K. et al. AIDS 19, 747–756 (2005).
Dean, M. et al. Science 273, 1856–1862 (1996).
Eugen-Olsen, J. et al. AIDS 11, 305–310 (1997).
Meyer, L. et al. AIDS 11, F73–F78 (1997).
Kuhmann, S.E. et al. J. Virol. 78, 2790–2807 (2004).
Maeda, K. et al. J. Virol. 78, 8654–8662 (2004).
Strizki, J.M. et al. Proc. Natl. Acad. Sci. USA 98, 12718–12723 (2001).
Takashima, K. et al. Antimicrob. Agents Chemother. 45, 3538–3543 (2001).
Dorr, P. et al. Antimicrob. Agents Chemother. (in the press).
Maggiolo, F. et al. HIV Clin. Trials 3, 371–378 (2002).
Adkins, J.C. & Noble, S. Drugs 56, 1055–1064 (1998).
Gulick, R.M. et al. N. Engl. J. Med. 337, 734–739 (1997).
McCallister, S. et al. J. Acquir. Immune Defic. Syndr. 35, 376–382 (2004).
Lalezari, J. et al. AIDS 19, 1443–1448 (2005).
Acknowledgements
We thank all the patients, study-site staff and Pfizer Global Research and Development staff for their contributions, which made this study possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.F. has received speaker fees and honoraria for expert advice from Pfizer.
A.L.P. has no competing financial interest or conflict of interest in the publication of this manuscript.
M.A.J. has received honoraria and traveling scholarships and has acted as an advisor to or received research funding from the following companies: Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline and Roche.
A.P. has no conflict of interest and no financial interests.
S.S. has been a member of several national and international scientific advisory boards founded by the pharmaceutical companies including GlaxoSmithKline, DuPont, Abbott, Gilead, Boehringer Ingelheim, Bristol-Meyers Squibb and Roche and has received honoraria for participation in the advisory boards and for lectures at satellite symposia.
A.I.M.H. has no competing financial interests other than the receipt grants for studies in the past and also for this study.
M.S.S. is a consultant to Pfizer and has grant support from Pfizer.
F.D.G. received a grant from Pfizer for this study. There is no additional support from Pfizer.
J.K.R. has received consultation or lecture fees from Abbott, Boehringer-Ingelheim, Bristol-Myers-Squibb, Gilead, GlaxoSmith-Kline, Pfizer, Roche and Schering-Plough.
B.J.D. is on Pfizer's speakers bureau but falls below the $10,000 amount and has no stock or other interests to declare.
T.M.J., C.M., J.F.S., C.R., S.A., I.T.J. and E.v.d.R. are employees of Pfizer Ltd.
M.Y. has no personal financial interests in Pfizer, has not been employed by Pfizer and has received no funding from Pfizer concerning this study.
Supplementary information
Supplementary Table 1
Mean maraviroc pharmacokinetic parameters on day 1 and day 10. (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Fätkenheuer, G., Pozniak, A., Johnson, M. et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med 11, 1170–1172 (2005). https://doi.org/10.1038/nm1319
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1319
This article is cited by
-
Interactions of Antiretroviral Drugs with Food, Beverages, Dietary Supplements, and Alcohol: A Systematic Review and Meta-analyses
AIDS and Behavior (2023)
-
CCR5 signaling promotes lipopolysaccharide-induced macrophage recruitment and alveolar developmental arrest
Cell Death & Disease (2021)
-
HIV-1 and human genetic variation
Nature Reviews Genetics (2021)
-
CNS Neurotoxicity of Antiretrovirals
Journal of Neuroimmune Pharmacology (2021)
-
The clinical potential of gene editing as a tool to engineer cell‐based therapeutics
Clinical and Translational Medicine (2020)