Key Points
-
In eukaryotic cells, the steady-state pH of intracellular compartments varies greatly, is tightly controlled and is an important determinant of their function.
-
In general, the cytoplasm tends to acidify as a result of catabolism and a negative membrane potential that drives the accumulation of H+ through cation channels and the loss of basic HCO3− through anion channels. Countering this acidification are intrinsic buffers (ionizable groups on amino acids, phosphates and other molecules) and HCO3−, which have a finite capacity, as well as distinct plasma membrane pH-regulatory transporters (for example, Na+–H+ exchangers and bicarbonate transporters) that finely control pH to keep it close to neutral — a level that is optimal for many protein interactions and cellular processes.
-
Some organelles, including the nucleus, endoplasmic reticulum and peroxisomes, seem to lack intrinsic pH-regulatory systems and instead seem highly permeable to H+ (or acid equivalents). Hence, these compartments readily equilibrate their luminal pH to levels found in the cytoplasm.
-
Organelles of the secretory and endocytic pathways are distinguished by their luminal acidity (pH 6.7–4.7), which is attained through the concerted actions of vacuolar H+–ATPases, 2 Cl−/1 H+ and Na+–H+ or K+–H+ exchangers. Progressive acidification of organelles along the secretory pathway is important for proper post-translational processing, sorting and transport of newly synthesized proteins. Likewise, graded acidification of vesicles along the endocytic pathway is essential for the recycling and/or degradation of internalized membrane proteins, fluid-phase solutes and entry of various microbial organisms.
-
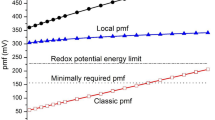
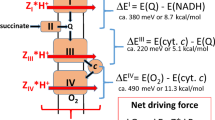
By contrast, the mitochondrial matrix is quite alkaline (pH 8.0) owing to H+ extrusion across the inner membrane by components of the electron transport chain. Together with the electrical potential (inside negative) generated by the electrogenic proton extrusion process, the transmembrane pH gradient constitutes a proton-motive force that is harnessed by the inner membrane H+-ATP synthase (F1F0-ATPase) to generate ATP from ADP and inorganic phosphate.
-
Many cellular processes are exquisitely sensitive to changes in the surrounding pH. Fluctuations in the H+ concentration in some cases act as a general permissive factor, whereas in other cases they act directly as a regulatory signal.
Abstract
Protons dictate the charge and structure of macromolecules and are used as energy currency by eukaryotic cells. The unique function of individual organelles therefore depends on the establishment and stringent maintenance of a distinct pH. This, in turn, requires a means to sense the prevailing pH and to respond to deviations from the norm with effective mechanisms to transport, produce or consume proton equivalents. A dynamic, finely tuned balance between proton-extruding and proton-importing processes underlies pH homeostasis not only in the cytosol, but in other cellular compartments as well.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Whitten, S. T., Garcia-Moreno, E. B. & Hilser, V. J. Local conformational fluctuations can modulate the coupling between proton binding and global structural transitions in proteins. Proc. Natl Acad. Sci. USA 102, 4282–4287 (2005).
Roos, A. & Boron, W. F. Intracellular pH. Physiol. Rev. 61, 296–434 (1981).
Sperelakis, N. Cell Physiology Source Book (Academic Press, San Diego, 1997).
Missner, A. et al. Carbon dioxide transport through membranes. J. Biol. Chem. 283, 25340–25347 (2008).
Grinstein, S., Furuya, W. & Biggar, W. D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J. Biol. Chem. 261, 512–514 (1986).
Forgac, M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nature Rev. Mol. Cell Biol. 8, 917–929 (2007).
Shin, J. M., Munson, K., Vagin, O. & Sachs, G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 457, 609–622 (2009).
Brown, D., Paunescu, T. G., Breton, S. & Marshansky, V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J. Exp. Biol. 212, 1762–1772 (2009).
Brett, C. L., Donowitz, M. & Rao, R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol., Cell Physiol. 288, C223–C239 (2005).
Orlowski, J. & Grinstein, S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr. Opin. Cell Biol. 19, 483–492 (2007).
Grinstein, S. et al. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: Assessment of effects on intracellular pH. EMBO J. 12, 5209–5218 (1993).
Biemesderfer, D. et al. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am. J. Physiol. 265, F736–F742 (1993).
Biemesderfer, D., Reilly, R. F., Exner, M., Igarashi, P. & Aronson, P. S. Immunocytochemical characterization of Na+-H+ exchanger isoform NHE-1 in rabbit kidney. Am. J. Physiol. 263, F833–F840 (1992).
Petrecca, K., Atanasiu, R., Grinstein, S., Orlowski, J. & Shrier, A. Subcellular localization of the Na+/H+ exchanger NHE1 in rat myocardium. Am. J. Physiol., Heart Circ. Physiol. 276, H709–H717 (1999).
Peti-Peterdi, J. et al. Macula densa Na+/H+ exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am. J. Physiol. 278, F452–F463 (2000).
Aronson, P. S. Kinetic properties of the plasma membrane Na-H exchanger. Annu. Rev. Physiol. 47, 545–560 (1985).
Fuster, D., Moe, O. W. & Hilgemann, D. W. Steady-state function of the ubiquitous mammalian Na/H exchanger (NHE1) in relation to dimer coupling models with 2Na/2H stoichiometry. J. Gen. Physiol. 132, 465–480 (2008).
Paris, S. & Pouysségur, J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+ J. Biol. Chem. 259, 10989–10994 (1984).
Otsu, K., Kinsella, J. L., Koh, E. & Froehlich, J. P. Proton dependence of the partial reactions of the sodium-proton exchanger in renal brush border membranes. J. Biol. Chem. 267, 8089–8096 (1992).
Olkhova, E., Hunte, C., Screpanti, E., Padan, E. & Michel, H. Multiconformation continuum electrostatics analysis of the NhaA Na+/H+ antiporter of Escherichia coli with functional implications. Proc. Natl Acad. Sci. USA 103, 2629–2634 (2006). The first in a series of detailed structural and mechanistic studies from this laboratory that define an electrostatic amino acid network, in which a Na+–H+ antiporter that links pH sensing with the cation binding site is crucial for pH activation of the transporter.
Olkhova, E., Kozachkov, L., Padan, E. & Michel, H. Combined computational and biochemical study reveals the importance of electrostatic interactions between the “pH sensor” and the cation binding site of the sodium/proton antiporter NhaA of Escherichia coli. Proteins 76, 548–559 (2009).
Olkhova, E., Padan, E. & Michel, H. The influence of protonation states on the dynamics of the NhaA antiporter from Escherichia coli. Biophys. J. 92, 3784–3791 (2007).
Hisamitsu, T., Yamada, K., Nakamura, T. Y. & Wakabayashi, S. Functional importance of charged residues within the putative intracellular loops in pH regulation by Na+/ H+ exchanger NHE1. FEBS J. 274, 4326–4335 (2007).
Wakabayashi, S., Hisamitsu, T., Pang, T. & Shigekawa, M. Mutations of Arg440 and Gly455/Gly456 oppositely change pH sensing of Na+/H+ exchanger 1. J. Biol. Chem. 278, 11828–11835 (2003).
Wakabayashi, S., Hisamitsu, T., Pang, T. & Shigekawa, M. Kinetic dissection of two distinct proton binding sites in Na+/H+ exchangers by measurement of reverse mode reaction. J. Biol. Chem. 278, 43580–43585 (2003).
Lacroix, J., Poet, M., Maehrel, C. & Counillon, L. A mechanism for the activation of the Na/H exchanger NHE-1 by cytoplasmic acidification and mitogens. EMBO Rep. 5, 91–96 (2004).
Monod, J., Wyman, J. & Changeux, J. P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Cuello, F., Snabaitis, A. K., Cohen, M. S., Taunton, J. & Avkiran, M. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to α1-adrenergic stimulation. Mol. Pharmacol. 71, 799–806 (2007).
Khaled, A. R. et al. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol. Cell. Biol. 21, 7545–7557 (2001).
Malo, M. E., Li, L. & Fliegel, L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids Ser770 and Ser771. J. Biol. Chem. 282, 6292–6299 (2007).
Takahashi, E. et al. p90 RSK is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J. Biol. Chem. 274, 20206–20214 (1999).
Tominaga, T., Ishizaki, T., Narumiya, S. & Barber, D. L. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 17, 4712–4722 (1998).
Yan, W. H., Nehrke, K., Choi, J. & Barber, D. L. The Nck-interacting kinase (NIK) phosphorylates the Na+-H+ exchanger NHE1 and regulates NHE1 activation by platelet-derived growth factor. J. Biol. Chem. 276, 31349–31356 (2001).
Aharonovitz, O. et al. Intracellular pH regulation by Na+/H+ exchange requires phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150, 213–224 (2000).
Bertrand, B., Wakabayashi, S., Ikeda, T., Pouysségur, J. & Shigekawa, M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J. Biol. Chem. 269, 13703–13709 (1994).
Denker, S. P., Huang, D. C., Orlowski, J., Furthmayr, H. & Barber, D. L. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol. Cell 6, 1425–1436 (2000).
Inoue, H. et al. Calcineurin homologous protein isoform 2 (CHP2), Na+/H+ exchangers-binding protein, is expressed in intestinal epithelium. Biol. Pharm. Bull. 26, 148–155 (2003).
Lehoux, S., Abe, J., Florian, J. A. & Berk, B. C. 14-3-3 binding to Na+/H+ exchanger isoform-1 is associated with serum-dependent activation of Na+/H+ exchange. J. Biol. Chem. 276, 15794–15800 (2001).
Li, X., Liu, Y., Alvarez, B. V., Casey, J. R. & Fliegel, L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry 45, 2414–2424 (2006).
Lin, X. & Barber, D. L. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc. Nat. Acad. Sci. USA 93, 12631–12636 (1996).
Mailander, J., Muller-Esterl, W. & Dedio, J. Human homolog of mouse tescalcin associates with Na+/H+ exchanger type-1. FEBS Lett. 507, 331–335 (2001).
Pang, T., Su, X., Wakabayashi, S. & Shigekawa, M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J. Biol. Chem. 276, 17367–17372 (2001).
Pang, T., Wakabayashi, S. & Shigekawa, M. Expression of calcineurin B homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na+/H+ exchanger. J. Biol. Chem. 277, 43771–43777 (2002). Identified the first bona fide , essential regulator of NHEs.
Meima, M. E., Mackley, J. R. & Barber, D. L. Beyond ion translocation: structural functions of the sodium-hydrogen exchanger isoform-1. Curr. Opin. Nephrol. Hypertens. 16, 365–372 (2007).
Denker, S. P. & Barber, D. L. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 159, 1087–1096 (2002). A seminal study, highlighting both a structural and a functional role for NHE1 in establishing polarity and directed migration of fibroblastic cells.
Stock, C. & Schwab, A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf.) 187, 149–157 (2006).
Hayashi, H. et al. Na+/H+ exchange and pH regulation in the control of neutrophil chemokinesis and chemotaxis. Am. J. Physiol., Cell Physiol. 294, C526–C534 (2008).
Patel, H. & Barber, D. L. A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J. Cell Biol. 169, 321–329 (2005).
Kapus, A., Grinstein, S., Wasan, S., Kandasamy, R. A. & Orlowski, J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells: ATP dependence, osmotic sensitivity and role in cell proliferation. J. Biol. Chem. 269, 23544–23552 (1994).
Pouysségur, J., Sardet, C., Franchi, A., L'Allemain, G. & Paris, S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc. Natl Acad. Sci. USA 81, 4833–4837 (1984). A classic paper identifying a crucial role for NHE1 in linking the regulation of intracellular pH to cell proliferation.
Putney, L. K. & Barber, D. L. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 278, 44645–44649 (2003).
Halestrap, A. & Meredith, D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 477, 619–628 (2004).
Vandenberg, J. I., Metcalfe, J. C. & Grace, A. A. Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ. Res. 72, 993–1003 (1993). Dissects the molecular mechanisms of pH regulation that are present in the intact heart.
Musa-Aziz, R., Chen, L. M., Pelletier, M. F. & Boron, W. F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl Acad. Sci. USA 106, 5406–5411 (2009).
Yang, B. et al. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J. Biol. Chem. 275, 2686–2692 (2000).
Cordat, E. & Casey, J. R. Bicarbonate transport in cell physiology and disease. Biochem. J. 417, 423–439 (2009).
Gross, E. et al. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J. Physiol. 531, 597–603 (2001). Provides evidence that the coupling stoichiometry for Na+–HCO 3− co-transport changes in a cell-type dependent manner.
Romero, M. F., Hediger, M. A., Boulpaep, E. L. & Boron, W. F. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387, 409–413 (1997). Reports on the first identification of a Na+–HCO 3− co-transporter gene, using an expression cloning approach.
Alper, S. L. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 212, 1672–1683 (2009).
Sterling, D. & Casey, J. R. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem. J. 344, 221–229 (1999).
Humphreys, B. D., Jiang, L., Chernova, M. N. & Alper, S. L. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am. J. Physiol., Cell Physiol. 267, C1295–C1307 (1994).
Gawenis, L. R. et al. Mice with a targeted disruption of the AE2 Cl−/HCO3− exchanger are achlorhydric. J. Biol. Chem. 279, 30531–30539 (2004).
Hug, M. J., Tamada, T. & Bridges, R. J. CFTR and bicarbonate secretion by epithelial cells. News Physiol. Sci. 18, 38–42 (2003).
Ko, S. B. et al. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 21, 5662–5672 (2002).
Mason, M. J., Smith, J. D., Garcia-Soto, J. J. & Grinstein, S. Internal pH-sensitive site couples Cl−-HCO3- exchange to Na+-H+ antiport in lymphocytes. Am. J. Physiol. 256, C428–C433 (1989).
Melvin, J. E., Park, K., Richardson, L., Schultheis, P. J. & Shull, G. E. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J. Biol. Chem. 274, 22855–22861 (1999).
Hoglund, P. et al. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nature Genet. 14, 316–319 (1996).
Goldfarb, D. S. in Nuclear Transport (ed. Kehlenbach, R.) (Landes Bioscience, Austin, 2009).
Paroutis, P., Touret, N. & Grinstein, S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 19, 207–215 (2004).
Kane, P. M. The long physiological reach of the yeast vacuolar H+-ATPase. J. Bioenerg. Biomembr. 39, 415–421 (2007).
Cipriano, D. J. et al. Structure and regulation of the vacuolar ATPases. Biochim. Biophys. Acta 1777, 599–604 (2008).
Barasch, J. & al-Awqati, Q. Defective acidification of the biosynthetic pathway in cystic fibrosis. J. Cell Sci. Suppl. 17, 229–233 (1993).
Seksek, O., Biwersi, J. & Verkman, A. S. Evidence against defective trans-Golgi acidification in cystic fibrosis. J. Biol. Chem. 271, 15542–15548 (1996).
Schapiro, F. B. & Grinstein, S. Determinants of the pH of the Golgi complex. J. Biol. Chem. 275, 21025–21032 (2000).
Numata, M. & Orlowski, J. Molecular cloning and characterization of a novel (Na+, K+)/H+ exchanger localized to the trans-Golgi network. J. Biol. Chem. 276, 17387–17394 (2001).
Ruetz, S., Lindsey, A. E., Ward, C. L. & Kopito, R. R. Functional activation of plasma membrane anion exchangers occurs in a pre-Golgi compartment. J. Cell Biol. 121, 37–48 (1993).
Shull, G. E. et al. Physiological functions of plasma membrane and intracellular Ca2+ pumps revealed by analysis of null mutants. Ann. N. Y. Acad. Sci. 986, 453–460 (2003).
Wu, M. M. et al. Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276, 33027–33035 (2001).
Weisz, O. A. Organelle acidification and disease. Traffic 4, 57–64 (2003).
Jentsch, T. J. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43, 3–36 (2008).
Di, A. et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nature Cell Biol. 8, 933–944 (2006).
Abad, M. F., Di Benedetto, G., Magalhaes, P. J., Filippin, L. & Pozzan, T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J. Biol. Chem. 279, 11521–11529 (2004).
Llopis, J., McCaffery, J. M., Miyawaki, A., Farquhar, M. G. & Tsien, R. Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl Acad. Sci. USA 95, 6803–6808 (1998).
Brierley, G. P., Baysal, K. & Jung, D. W. Cation transport systems in mitochondria: Na+ and K+ uniports and exchangers. J. Bioenerg. Biomembr. 26, 519–526 (1994).
Crompton, M. & Heid, I. The cycling of calcium, sodium, and protons across the inner membrane of cardiac mitochondria. Eur. J. Biochem. 91, 599–608 (1978).
Garlid, K. D., Sun, X., Paucek, P. & Woldegiorgis, G. Mitochondrial cation transport systems. Methods Enzymol. 260, 331–348 (1995).
Gunter, T. E., Gunter, K. K., Sheu, S. S. & Gavin, C. E. Mitochondrial calcium transport: physiological and pathological relevance. Am. J. Physiol. 267, C313–C339 (1994).
McCormack, J. G., Halestrap, A. P. & Denton, R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425 (1990).
Moreno-Sanchez, R. Inhibition of oxidative phosphorylation by a Ca2+ -induced diminution of the adenine nucleotide translocator. Biochim. Biophys. Acta 724, 278–285 (1983).
Yamada, E. W. & Huzel, N. J. Calcium-binding ATPase inhibitor protein of bovine heart mitochondria. Role in ATP synthesis and effect of Ca2+. Biochemistry 28, 9714–9718 (1989).
Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995).
Matsuyama, S., Llopis, J., Deveraux, Q. L., Tsien, R. Y. & Reed, J. C. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nature Cell Biol. 2, 318–325 (2000). This paper identifies disruptions in mitochondrial and cytoplasmic pH homeostasis as important early events in mitochondrial-dependent apoptosis.
Matsuyama, S., Xu, Q., Velours, J. & Reed, J. C. The mitochondrial F0 F1 -ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell 1, 327–336 (1998).
Nicholls, D. et al. Apoptosis and the laws of thermodynamics. Nature Cell Biol. 2, E172–E173 (2000).
Thangaraju, M., Sharma, K., Liu, D., Shen, S. H. & Srikant, C. B. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 59, 1649–1654 (1999).
Lupescu, A. et al. Inhibition of Na+/H+ exchanger activity by parvovirus B19 protein NS1. Cell Physiol. Biochem. 23, 211–220 (2009).
Schneider, D. et al. Intracellular acidification by inhibition of the Na+/H+-exchanger leads to caspase-independent death of cerebellar granule neurons resembling paraptosis. Cell Death Differ. 11, 760–770 (2004).
Hoepfner, D., Schildknegt, D., Braakman, I., Philippsen, P. & Tabak, H. F. Contribution of the endoplasmic reticulum to peroxisome formation. Cell 122, 85–95 (2005).
Titorenko, V. I. & Rachubinski, R. A. Spatiotemporal dynamics of the ER-derived peroxisomal endomembrane system. Int. Rev. Cell. Mol. Biol. 272, 191–244 (2009).
Reddy, J. K. & Mannaerts, G. P. Peroxisomal lipid metabolism. Annu. Rev. Nutr. 14, 343–370 (1994).
Dansen, T. B., Wirtz, K. W., Wanders, R. J. & Pap, E. H. Peroxisomes in human fibroblasts have a basic pH. Nature Cell Biol. 2, 51–53 (2000).
Jankowski, A. et al. In situ measurements of the pH of mammalian peroxisomes using the fluorescent protein pHluorin. J. Biol. Chem. 276, 48748–48753 (2001).
Drago, I., Giacomello, M., Pizzo, P. & Pozzan, T. Calcium dynamics in the peroxisomal lumen of living cells. J. Biol. Chem. 283, 14384–14390 (2008).
Srivastava, J., Barber, D. L. & Jacobson, M. P. Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 22, 30–39 (2007).
Pouysségur, J., Franchi, A., L'Allemain, G. & Paris, S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 190, 115–119 (1985).
Schelling, J. R. & Abu Jawdeh, B. G. Regulation of cell survival by Na+/H+ exchanger-1. Am. J. Physiol., Renal Physiol. 295, F625–F632 (2008).
Bierman, A., Cragoe, E. J., Jr, de Laat, S. W. & Moolenaar, W. H. Bicarbonate determines cytoplasmic pH and suppresses mitogen-induced alkalinization in fibroblastic cells. J. Biol. Chem. 263, 15253–15256 (1988).
Spitzer, K. W., Skolnick, R. L., Peercy, B. E., Keener, J. P. & Vaughan-Jones, R. D. Facilitation of intracellular H+ ion mobility by CO2/HCO3− in rabbit ventricular myocytes is regulated by carbonic anhydrase. J. Physiol. 541, 159–167 (2002).
Vaughan-Jones, R. D., Peercy, B. E., Keener, J. P. & Spitzer, K. W. Intrinsic H+ ion mobility in the rabbit ventricular myocyte. J. Physiol. 541, 139–158 (2002). Reveals the surprisingly slow rate of H+ diffusion in the cytosol.
Stewart, A. K., Boyd, C. A. & Vaughan-Jones, R. D. A novel role for carbonic anhydrase: cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J. Physiol. 516, 209–217 (1999).
Stock, C. et al. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol. Biochem. 20, 679–686 (2007).
Stock, C. & Schwab, A. Protons make tumor cells move like clockwork. Pflugers Arch. 458, 981–992 (2009).
Simons, M. et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nature Cell Biol. 11, 286–294 (2009).
Beg, A. A., Ernstrom, G. G., Nix, P., Davis, M. W. & Jorgensen, E. M. Protons act as a transmitter for muscle contraction in C. elegans. Cell 132, 149–160 (2008). Elegant studies of C. elegans indicate that H+ ions, secreted by an intestinal NHE, act on a proton-gated cation channel in muscle cells to signal muscle contraction, which provides evidence for a role of extracellular protons as a neurotransmitter.
Waldmann, R. et al. H+ -gated cation channels. Ann. N. Y. Acad. Sci. 868, 67–76 (1999).
DeVries, S. H. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32, 1107–1117 (2001).
Pastorekova, S., Parkkila, S., Pastorek, J. & Supuran, C. T. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J. Enzyme Inhib. Med. Chem. 19, 199–229 (2004).
Obara, M., Szeliga, M. & Albrecht, J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem. Int. 52, 905–919 (2008).
Vaughan-Jones, R. D., Spitzer, K. W. & Swietach, P. Intracellular pH regulation in heart. J. Mol. Cell Cardiol. 46, 318–331 (2008).
Hara-Chikuma, M., Wang, Y., Guggino, S. E., Guggino, W. B. & Verkman, A. S. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem. Biophys. Res. Commun. 329, 941–946 (2005).
Piwon, N., Gunther, W., Schwake, M., Bosl, M. R. & Jentsch, T. J. ClC-5 Cl− -channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408, 369–373 (2000).
Kornak, U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001).
Kasper, D. et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091 (2005).
Poet, M. et al. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl Acad. Sci. USA 103, 13854–13859 (2006).
Kornak, U. et al. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nature Genet. 40, 32–34 (2008).
Acknowledgements
Original work from the authors' laboratories is supported by the Heart and Stroke Foundation of Canada, the Kidney Foundation and the Canadian Institutes of Health Research. S.G holds the Pitblado Chair in Cell Biology. J.R.C is a scientist of the Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Proton-motive force
-
(ψH+). The driving force for proton (or equivalent) movement, consisting of the proton concentration gradient and the transmembrane electrical potential.
- pH buffering capacity
-
A measure of the ability of a solution to withstand changes in pH. It is defined as β = dn/dpH, where n is the number of acid or base equivalents that need to be added to alter pH.
- pKa
-
The acid dissociation constant. A quantitative measure of the tendency of an acid to dissociate in solution. It is calculated as pKa = −log10Ka, where Ka = [A−][H+]/[HA] and [A−], [H+] and [HA] are the concentration of the dissociated acid, protons and the undissociated (protonated) acid, respectively.
- Na+–K+-ATPase
-
A ubiquitous plasmalemmal enzyme that uses ATP to extrude 3 Na+ ions in exchange for 2 K+ ions. Also known as the Na+–K+-pump or simply the Na-pump.
- Hill coefficient
-
A measure of the cooperativity of a binding process. It is calculated by applying the Hill equation, which relates the fraction of filled ligand-binding sites to the ligand concentration.
- Aquaporin water channel
-
One of a family of proteins that facilitate the passage of water across biological membranes.
- Phagosome
-
A vacuole that forms inside cells following the engulfment of large (≥0.5 μm) particles by a receptor-mediated, actin-driven process.
Rights and permissions
About this article
Cite this article
Casey, J., Grinstein, S. & Orlowski, J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11, 50–61 (2010). https://doi.org/10.1038/nrm2820
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2820
This article is cited by
-
Amelioration of obsessive-compulsive disorder by intracellular acidification of cortical neurons with a proton pump inhibitor
Translational Psychiatry (2024)
-
Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion
Nature Immunology (2024)
-
Carnosine helps cancer cells to evade immune surveillance by regulating intracellular pH
Nature Immunology (2024)
-
Lysosome blockade induces divergent metabolic programs in macrophages and tumours for cancer immunotherapy
Journal of Experimental & Clinical Cancer Research (2023)
-
Isomerization of bioactive acylhydrazones triggered by light or thiols
Nature Chemistry (2023)