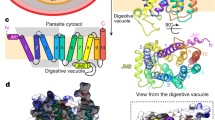
Abstract
Plasmodium falciparum dihydrofolate reductase–thymidylate synthase (PfDHFR-TS) is an important target of antimalarial drugs. The efficacy of this class of DHFR-inhibitor drugs is now compromised because of mutations that prevent drug binding yet retain enzyme activity. The crystal structures of PfDHFR-TS from the wild type (TM4/8.2) and the quadruple drug-resistant mutant (V1/S) strains, in complex with a potent inhibitor WR99210, as well as the resistant double mutant (K1 CB1) with the antimalarial pyrimethamine, reveal features for overcoming resistance. In contrast to pyrimethamine, the flexible side chain of WR99210 can adopt a conformation that fits well in the active site, thereby contributing to binding. The single-chain bifunctional PfDHFR-TS has a helical insert between the DHFR and TS domains that is involved in dimerization and domain organization. Moreover, positively charged grooves on the surface of the dimer suggest a function in channeling of substrate from TS to DHFR active sites. These features provide possible approaches for the design of new drugs to overcome antifolate resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Breman, J.G. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64, 1–11 (2001).
Ferone, R. Folate metabolism in malaria. Bull. World Health Organ. 55, 291–298 (1977).
Cowman, A.F., Morry, M.J., Biggs, B.A., Cross, G.A.M. & Foote, S.J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase–thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85, 9109–9113 (1988).
Peterson, D.S., Walliker, D. & Wellems, T.E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 85, 9114–9118 (1988).
Foote, S.J., Galatis, D. & Cowman, A.F. Amino acids in the dihydrofolate reductase–thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc. Natl. Acad. Sci. USA 87, 3014–3017 (1990).
Peterson, D.S., Milhous, W.K. & Wellems, T.E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 87, 3018–3022 (1990).
Sirawaraporn, W., Sathitkul, T., Sirawaraporn, R., Yuthavong, Y. & Santi, D.V. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 94, 1124–1129 (1997).
Childs, G.E. & Lambros, C. Analogues of N-benzyloxydihydrotriazines: in vitro antimalarial activity against Plasmodium falciparum. Ann. Trop. Med. Parasitol. 80, 177–181 (1986).
Canfield, C.J. et al. PS-15: a potent, orally active antimalarial from a new class of folic acid antagonists. Am. J. Trop. Med. Hyg. 49, 121–126 (1993).
Ferone, R. & Roland, S. Dihydrofolate reductase: thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc. Natl. Acad. Sci. USA 77, 5802–5806 (1980).
Ivanetich, K.M. & Santi, D.V. Bifunctional thymidylate synthase–dihydrofolate reductase in protozoa. FASEB J. 4, 1591–1597 (1990).
Bzik, D.J., Li, W.-B., Horii, T. & Inselburg, J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase–thymidylate synthase gene. Proc. Natl. Acad. Sci. USA 84, 8360–8364 (1987).
Hyde, J.E. The dihydrofolate reductase–thymidylate synthase gene in the drug resistance of malaria parasites. Pharmacol. Ther. 48, 45–59 (1990).
Knighton, D.R. et al. Structure of and kinetic channelling in bifunctional dihydrofolate reductase–thymidylate synthase. Nat. Struct. Biol. 1, 186–194 (1994).
Lemcke, T., Christensen, I.T. & Jorgensen, F.S. Towards an understanding of drug resistance in malaria: three-dimensional structure of Plasmodium falciparum dihydrofolate reductase by homology building. Bioorg. Med. Chem. 7, 1003–1011 (1999).
Rastelli, G. et al. Interactions of pyrimethamine, cycloguanil, WR99210 and their analogues with Plasmodium falciparum dihydrofolate reductase: structural basis of antifolate resistance. Bioorg. Med. Chem. 8, 1117–1128 (2000).
Warhurst, D.C. Antimalarial drug discovery: development of inhibitors of dihydrofolate reductase active in drug resistance. Drug Discovery Today 3, 538–546 (1998).
McKie, J.H. et al. Rational drug design approach for overcoming drug resistance: application to pyrimethamine resistance in malaria. J. Med. Chem. 41, 1367–1370 (1998).
Zhang, K. & Rathod, P.K. Divergent regulation of dihydrofolate reductase between malaria parasite and human host. Science 296, 545–547 (2002).
Hardy, L.W. et al. Atomic structure of thymidylate synthase: target for rational drug design. Science 235, 448–455 (1987).
Carreras, C.W. & Santi, D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 64, 721–762 (1995).
Shallom, S., Zhang, K., Jiang, L. & Rathod, P.K. Essential protein–protein interactions between Plasmodium falciparum thymidylate synthase and dihydrofolate reductase domains. J. Biol. Chem. 274, 37781–37786 (1999).
Garrett, C.E. et al. A bifunctional thymidylate synthetase–dihydrofolate reductase in protozoa. Mol. Biochem. Parasitol. 11, 257–265 (1984).
Cella, R., Carbonera, D., Orsi, R., Ferri, G. & Iadarola, P. Proteolytic and partial sequencing studies of the bifunctional dihydrofolate reductase–thymidylate synthase from Daucus carota. Plant Mol. Biol. 16, 975–982 (1991).
Lazar, G., Zhang, H. & Goodman, H.M. The origin of the bifunctional dihydrofolate reductase-thymidylate synthase isogenes of Arabidopsis thaliana. Plant J. 3, 657–668 (1993).
Meek, T.D., Garvey, E.P. & Santi, D.V. Purification and characterization of the bifunctional thymidylate synthetase–dihydrofolate reductase from methotrexate-resistant Leishmania tropica. Biochemistry 24, 678–686 (1985).
Elcock, A.H., Potter, M.J., Matthews, D.A., Knighton, D.R. & McCammon, J.A. Electrostatic channeling in the bifunctional enzyme dihydrofolate reductase–thymidylate synthase. J. Mol. Biol. 262, 370–374 (1996).
Chayen, N.E., Shaw Stewart, P.D., Maeder, D.L. & Blow, D.M. An automated system for micro-batch protein crystallization and screening. J. Appl. Crystallogr. 23, 297–302 (1990).
D'Arcy, A., Elmore, C., Stihle, M. & Johnston, J.E. A novel approach to crystallizing proteins under oil. J. Cryst. Growth 168, 175–180 (1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Pflugrath, J.W. The finer things in X-ray diffraction data collection. Acta Crystallogr. D 55, 1718–1725 (1999).
Collaborative Computational Project, Number 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Sotelo-Mundo, R.R. et al. Crystal structures of rat thymidylate synthase inhibited by tomudex, a potent anticancer drug. Biochemistry 38, 1087–1094 (1999).
Whitlow, M. et al. X-ray crystallographic studies of Candida albicans dihydrofolate reductase high-resolution structures of the holoenzyme and an inhibited ternary complex. J. Biol. Chem. 272, 30289–30298 (1997).
Sawaya, M.R. & Kraut, J. Loop and subdomain movements in the mechanism of Escherichia coli dihydrofolate reductase: crystallographic evidence. Biochemistry 36, 586–603 (1997).
McTigue, M.A., Davies, J.F. II, Kaufman, B.T. & Kraut, J. Crystal structure of chicken liver dihydrofolate reductase complexed with NADP+ and biopterin. Biochemistry 31, 7264–7273 (1992).
Pieper, U., Kapadia, G., Mevarech, M. & Herzberg, O. Structural features of halophilicity derived from the crystal structure of dihydrofolate reductase from the Dead Sea halophilic archaeon, Haloferax volcanii. Structure 6, 75–88 (1998).
Cody, V. et al. Comparison of ternary crystal complexes of F31 variants of human dihydrofolate reductase with NADPH and a classical antitumor furopyrimidine. Anticancer Drug Des. 13, 307–315 (1998).
Bolin, J.T., Filman, D.J., Matthews, D.A., Hamlin, R.C. & Kraut, J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 Å resolution. I. General features and binding of methotrexate. J. Biol. Chem. 257, 13650–13662 (1982).
Li, R. et al. Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs. J. Mol. Biol. 295, 307–323 (2000).
Champness, J.N. et al. The structure of Pneumocystis carinii dihydrofolate reductase to 1.9 Å resolution. Structure 2, 915–924 (1994).
Dams, T. et al. The crystal structure of dihydrofolate reductase from Thermotoga maritima: molecular features of thermostability. J. Mol. Biol. 297, 659–672 (2000).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A.T. et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A. A set of averaging programs. in Molecular Replacement (eds. Dodson, E.J., Gover, S., and Wolf, W.) 91–105 (SERC Daresbury Laboratory, Warrington, UK; 1992).
Kleywegt, G.J. & Jones, T.A. xdlMAPMAN and xdlDATAMAN-programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Crystallogr. D 52, 826–828 (1996).
Kleywegt, G.J. & Jones, T.A. Template convolution to enhance or detect structural features in macromolecular electron-density maps. Acta Crystallogr. D 53, 179–185 (1997).
Perrakis, A., Sixma, T.K., Wilson, K.S. & Lamzin, V.S. wARP: improvement and extension of crystallographic phases by weighted averaging of multiple refined dummy atomic models. Acta Crystallogr. D 53, 448–455 (1997).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Beverley, S.M., Ellenberger, T.E. & Cordingley, J.S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc. Natl. Acad. Sci. USA 83, 2584–2588 (1986).
Eldin de Pecoulas, P., Basco, L.K., Tahar, R., Ouatas, T. & Mazabraud, A. Analysis of the Plasmodium vivax dihydrofolate reductase–thymidylate synthase gene sequence. Gene 211, 177–185 (1998).
Cowman, A.F. & Lew, A.M. Antifolate drug selection results in duplication and rearrangement of chromosome 7 in Plasmodium chabaudi. Mol. Cell. Biol. 9, 5182–1588 (1989).
van Dijk, M.R., McConkey, G.A., Vinkenoog, R., Waters, A.P. & Janse, C.J. Mechanisms of pyrimethamine resistance in two different strains of Plasmodium berghei. Mol. Biochem. Parasitol. 68, 167–171 (1994).
Cheng, Q. & Saul, A. The dihydrofolate reductase domain of rodent malarias: point mutations and pyrimethamine resistance. Mol. Biochem. Parasitol. 65, 361–363 (1994).
Higgins, D. et al. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Koradi, R., Billeter, M. & Wüthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics 14, 51–55 (1996).
Acknowledgements
We thank the Wellcome Trust for the Collaborative Research Grant to Y.Y. and M.W. The present work was also partly supported by grants from the EU, Medicines for Malaria Venture (MMV), the Special Programme for Research and Training in Tropical Diseases (TDR)/United Nations Development Programme/the World Bank/the World Health Organization and Thailand Tropical Diseases Research (T2) Programmes to Y.Y. and S.K. and from the TDR to W.S. We are grateful to CCLRC, ESRF, EMBL and NSLS for use of synchrotron facilities and to W.N. Lipscomb for his suggestions and comments on the manuscript. We also thank S. Thaithong, Department of Biology, Faculty of Science, Chulalongkorn University (TM4/8.2 and K1 CB1), and D. Kyle through MR4 (V1/S) for sources of the parasite strains.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yuvaniyama, J., Chitnumsub, P., Kamchonwongpaisan, S. et al. Insights into antifolate resistance from malarial DHFR-TS structures. Nat Struct Mol Biol 10, 357–365 (2003). https://doi.org/10.1038/nsb921
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb921
This article is cited by
-
Babesia duncani multi-omics identifies virulence factors and drug targets
Nature Microbiology (2023)
-
Microwave synthesis and antimalarial screening of novel 4-amino benzoic acid (PABA)-substituted pyrimidine derivatives as Plasmodium falciparum dihydrofolate reductase inhibitors
3 Biotech (2022)
-
Dihydrofolate reductase, thymidylate synthase, and serine hydroxy methyltransferase: successful targets against some infectious diseases
Molecular Biology Reports (2022)
-
Polymerase-guided base editing enables in vivo mutagenesis and rapid protein engineering
Nature Communications (2021)
-
A review of antimalarial activity of two or three nitrogen atoms containing heterocyclic compounds
Medicinal Chemistry Research (2020)